Is Matter Around Us Pure? Class 9 Notes Science Chapter 2
| Table of contents |

|
| Introduction |

|
| What Is a Mixture? |

|
| What is a Solution? |

|
| What is a Suspension? |

|
| What is a Colloidal solution? |

|
| Physical and Chemical changes |

|
| What are the Types of Pure Substances? |

|
| Mixture |

|
Introduction
Have you ever wondered if the water you drink, the air you breathe, or even the food you eat is completely pure? The chapter delves into the fascinating world of matter, where we explore how everything around us, from a simple sugar cube to the air we inhale, is made up of pure substances or mixtures. You'll discover how mixtures can be separated into their components, and how pure substances are the building blocks of everything we see and use!
What Is a Mixture?
Mixtures are constituted by more than one kind of pure form of matter, known as a substance. For example, sea water, minerals, soil etc., are all mixtures.
- When we say that something is pure, it means that all the constituent particles of that substance are the same in their chemical nature. A pure substance always consists of a single type of particle.
- When we look around, we can see that most of the matter around us exist as mixtures of two or more pure components.
 Examples of Mixtures
Examples of Mixtures
- Dissolved sodium chloride can be separated from water by the physical process of evaporation but sodium chloride cannot be separated into sodium and chlorine by physical means.
Types of mixtures
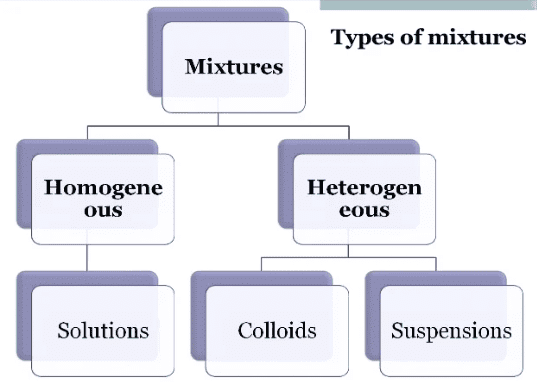
Depending upon the nature of components that form a mixture, we can have different types of mixtures.
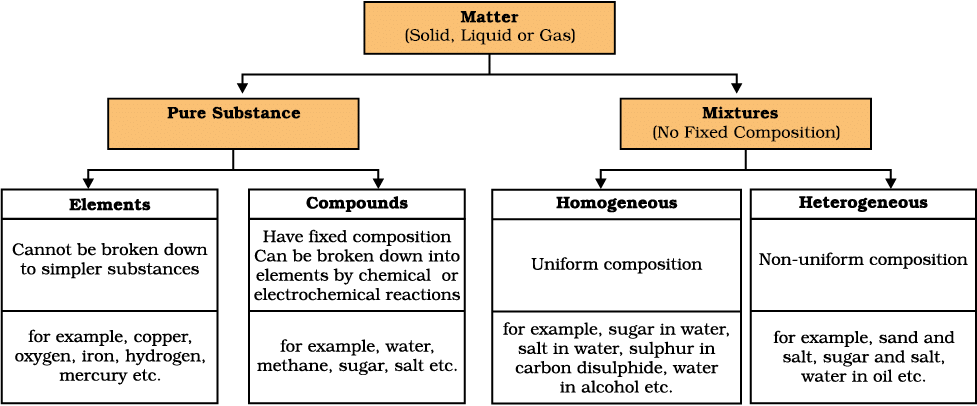
- Homogeneous mixtures: Mixtures which have a uniform composition throughout, are called homogeneous mixtures. For example, salt in water and sugar in water.
- Heterogeneous mixtures: Mixtures which contain physically distinct parts and have non-uniform composition are called heterogeneous mixtures. For example, mixture of sodium chloride and iron filings, salt and sulphur.
Activity: Perform an activity to differentiate between solution, suspension and colloidal solution.
Procedure:
- Distribute the following samples to four groups A, B, C and D of a class.
- A few crystals of copper sulphate to group A.
- One spatula is full of copper sulphate to group B.
- Chalk powder to group C.
- A few drops of milk or ink to group D.
- Ask each group to add the sample to water and stir using a glass rod.
- Direct a beam of light from a torch through the beakers.
- Leave the mixture undisturbed for a few minutes.
- Filter the mixtures
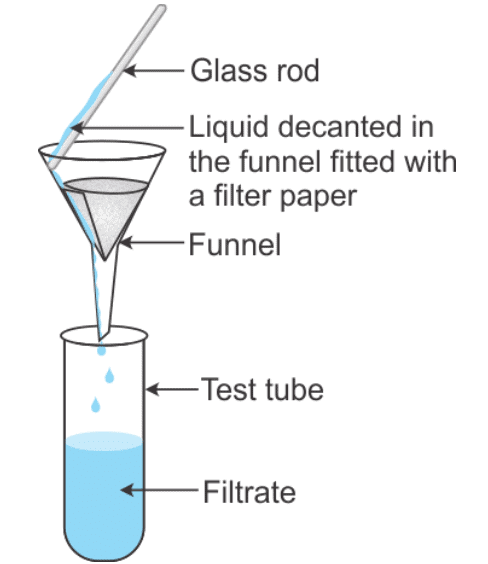
 Solution, Suspension and Colloidal Solution
Solution, Suspension and Colloidal Solution
Observations:
- We observe that groups A and B get a clear solution of copper sulphate although with different colour density.
- Group C get a suspension of chalk, which on filtration gives a residue of chalk on the filter paper and clear filtrate containing water.
- Group D get a colloidal solution of milk. The solution in this case is not transparent. But no suspension is obtained here and on filtration, no residue is obtained on the filter paper.
Conclusion:
- Group A and B created homogeneous mixtures with uniform composition, while Groups C and D made heterogeneous mixtures with distinct parts.
- This activity illustrates the difference between homogeneous and heterogeneous mixtures based on composition and appearance.
What is a Solution?
A solution is a homogeneous mixture of two or more substances. Lemonade and soda water are example of solutions.
A solution is not necessarily a liquid containing a solid, liquid or gas dissolved in it. Solid solution (alloys) and gaseous solution are also possible.
Alloys
Alloys are homogeneous mixtures of metals and cannot be separated into their components by physical methods. For example, brass is a mixture of approximately 30% zinc and 70% copper.

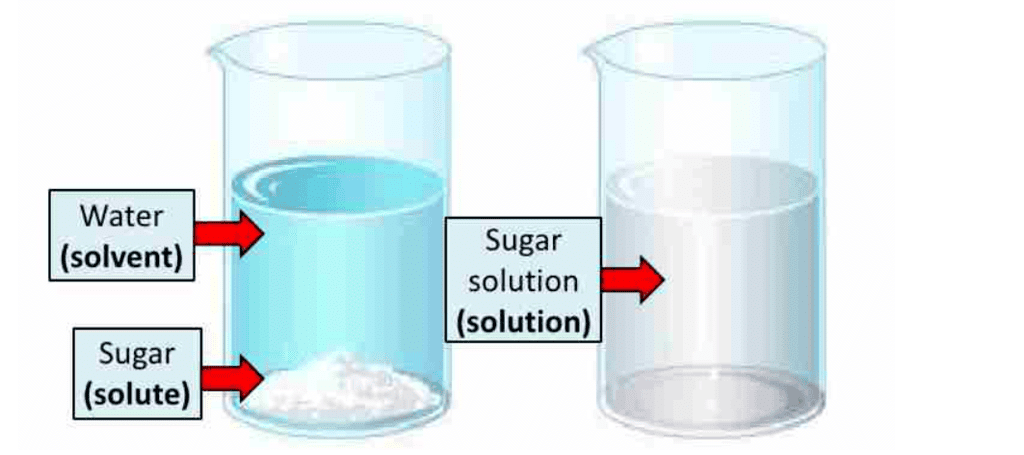
Solvent and solute
Solvent and solute are the components of the solution.
- Solvent: The component that dissolves the other component in it is the solvent.
- Solute: The component that is dissolved in the solvent is called solute.
For example, Tincture of iodine is a solution of iodine in alcohol. Aerated drinks like soda water are solutions of carbon dioxide as solute and water as solvent. Air is a mixture of a gas in a gas. The two major components of air are nitrogen (78%) and oxygen (21%).
Note: Generally solute is present in smaller quantity and solvent is present in greater quantity. For example, we have a solution of sugar in water in which case sugar is solute and water is the solvent.
Properties of a solution
- A solution is a homogeneous mixture.
- Particles of a solution are smaller than 1 nm (10-9metre) in diameter. Therefore, they cannot be seen by naked eye.
- Because of small size, they do not scatter light.
- Solute particles cannot be separated from the mixture by filtration.
Concentration of the solution
Concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution.
- A concentrated solution contains a large concentration of the solute in the solvent while a dilute solution contains a small concentration of the solute in the solvent.
- Mass by mass percentage of a solution
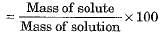
- Mass by volume percentage of a solution
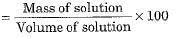
Saturated solution
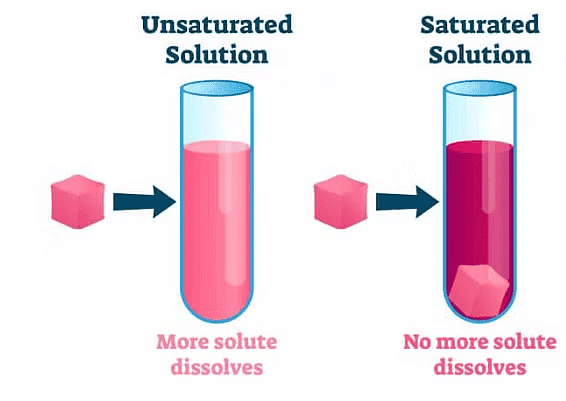
At any particular temperature, a solution that has dissolved as much solute as it is capable of dissolving, is called saturated solution.
No more solute can be dissolved in the saturated solution at a given temperature.
Solubility: The amount of solute present in a saturated solution at a given temperature is called its solubility
Unsaturated solution
If the amount of solute contained in a solution is less than saturation level, it is called unsaturated solution.
Different substances in a given solvent have different solubilities at the same temperature.
What is a Suspension?
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of medium.
- Particles of a suspension are visible to the naked eye.
- For example, chalk powder in water.
Properties of a suspension
- Suspension is a heterogeneous mixture.
- Particles of suspension can be seen with a naked eye.
- Particles of a suspension scatter light passing through it and make its path visible.
- Solute particles in a suspension settle down after some time when kept undisturbed.
- Components of a suspension can be separated by the process of filtration.
What is a Colloidal solution?
A colloidal solution is a heterogeneous mixture in which the solute particles do not settle down but remain suspended.
- Here the particle size of the solute is between 1 nm to 100 nm.
- Colloidal particles cannot be seen with a naked eye but they scatter light thus making the path of light visible.
- For example, milk and starch solution.
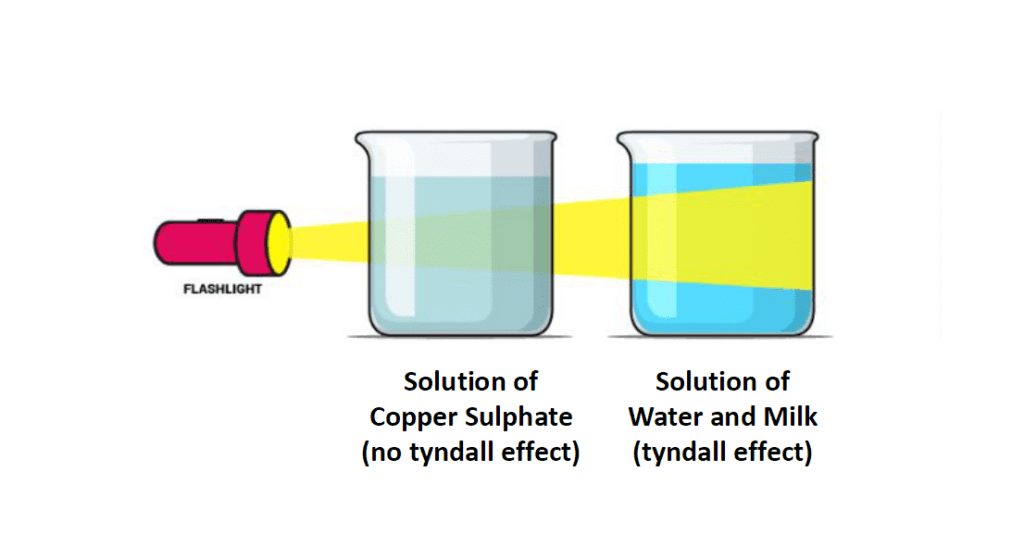 Solution of Copper Sulphate does not show Tyndall Effect, Mixture of Water and Milk shows Tyndall Effect
Solution of Copper Sulphate does not show Tyndall Effect, Mixture of Water and Milk shows Tyndall Effect
Properties of colloidal solutions
- A colloidal solution is a heterogeneous mixture.
- The particles of a colloid cannot be seen with a naked eye.
- Colloidal particles scatter light.
- Colloidal particles do not settle down when left undisturbed.
- Colloidal particles cannot be separated from the mixture by the process of filtration.
 |
Download the notes
Chapter Notes: Is Matter Around Us Pure
|
Download as PDF |
Dispersed phase and dispersion medium
- These are the components of a colloidal solution.
- The solute-like component in a colloidal solution are dispersed phase and the solvent like component in a colloidal solution is dispersed medium.
Some common examples of colloids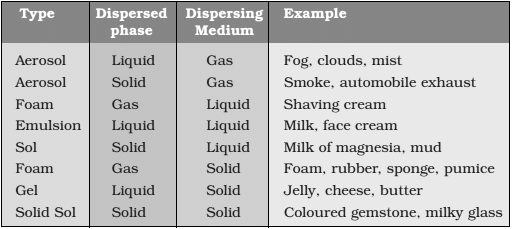
Physical and Chemical changes
A change which occurs without a change in composition and chemical nature of the substance is called physical change.
- Here a change only in physical properties of the substance takes place.
- Properties like colour, hardness, rigidity, fluidity, density, melting point and boiling point are known as physical properties.
- Melting of ice or boiling of water is a physical change because ice, water and water vapours are chemically the same substance i.e., H20.
A change of materials into another, new materials with different properties and one or more than one new substances are formed is called chemical change.
- Burning is a chemical change.
- During this process, one substance reacts with another substance to undergo a change in chemical composition.
- During burning of candle, actually both physical and chemical changes take place.
- The physical change involves the melting of wax and the chemical change involves the burning of wax into carbon dioxide and water.

What are the Types of Pure Substances?
On the basis of their chemical composition, substances can be classified either as elements or compounds.
Elements
- Lavoisier, a French chemist defined an element as the basic form of matter that cannot be broken down into simpler substances by chemical reactions.
- Elements can be divided into the following main threetypes of substances:
- Metals.
- Non-metals.
- Metalloids.
 Metals show the following properties
Metals show the following properties
- They have a Lustre.
- They have silvery-grey or golden-yellow colour.
- They conduct heat and electricity.
- They are ductile that means they can be drawn into thin wires.
- They are malleable. That means they can be beaten into thin sheets.
- They are sonorous i.e., they make a ringing sound when hit.
- Examples of metals are gold, silver, copper, iron, sodium, etc.
Non-metals show the following properties
- They display a variety of colours.
- They are poor conductors of heat and electricity.
- They are not lustrous, sonorous or malleable.
- Examples of non-metals are oxygen, iodine, carbon, etc.
- Some elements have intermediate properties between those of metals and non-metals.
- They are called metalloids.
- Examples of metalloids are boron, silicon and germanium.
Some facts about elements
- The number of elements known at present is more than 100. Ninety two elements are naturally occurring and the rest are man-made.
- Majority of the elements are solids.
- Eleven elements are in gaseous state at room temperature.
- Two elements are liquid at room temperature - mercury and bromine.
- Elements gallium and cesium become liquid at a temperature slightly above room temperature (303 K).
Compounds
A compound is a substance composed of two or more elements chemically combined with one another in a fixed proportion.
Activity - Exploring the Properties of Iron and Sulphur Mixture.
Materials required: Crushed iron filings, sulphur, china dish, burner.
Procedure:
- Divide the class into two groups.
- Provide each group with 5 g of iron filings and 3 g of sulphur powder in a china dish.
Group I:
- Mix and crush iron filings and sulphur powder together.
Group II:
- Mix and crush iron filings and sulphur powder together.
- Heat the mixture strongly until it becomes red hot.
- Remove from flame and let the mixture cool down.
Both Groups:
- Check for magnetism in the material obtained by bringing a magnet near it.
- Compare the texture and color of the material obtained by both groups.
- Add carbon disulphide to one part of the material obtained, stir well, and filter.
- Add dilute sulphuric acid or dilute hydrochloric acid to another part of the material obtained. (Note: Teacher supervision is necessary for this activity).
- Perform all the above steps with iron and sulphur separately.
Observation:
Upon heating, iron and sulfur react chemically to form a compound. This compound has different properties from the original elements, indicating a chemical change. The mixture of iron and sulfur before heating shows the individual properties of both substances, but once heated, a new substance with distinct properties is created.Conclusion
- When iron and sulfur are mixed and heated:
- Group I demonstrates a physical change, resulting in a mixture with similar properties to the individual substances (iron and sulfur).
- Group II exhibits a chemical change, where iron and sulfur react to form a compound with different properties.
This experiment highlights the differences between physical and chemical changes, as well as the concepts of mixtures and compounds in chemistry.
Mixture
If we simply mix iron filings with powdered sulphur and grind them together (no heating), we. obtain a mixture.
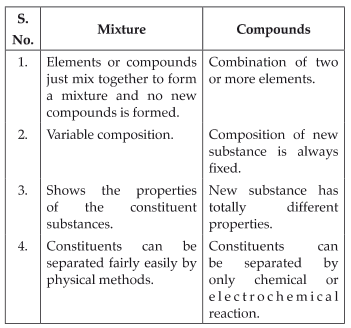
Comparison between mixtures and compounds
Table: Mixtures and Compounds
- We can summarise the physical and chemical nature of matter as under:
|
84 videos|394 docs|61 tests
|
FAQs on Is Matter Around Us Pure? Class 9 Notes Science Chapter 2
| 1. What is the difference between a mixture and a pure substance? |  |
| 2. How can you differentiate between a solution and a suspension? |  |
| 3. What are colloidal solutions and how do they differ from solutions? |  |
| 4. What are some examples of physical changes and chemical changes? |  |
| 5. What are the different types of pure substances? |  |





















