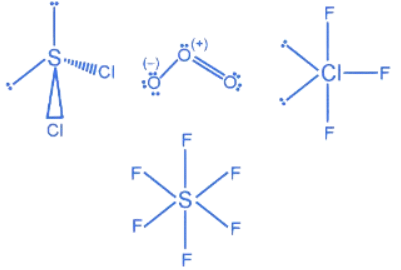
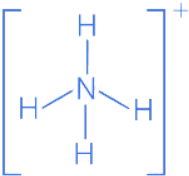
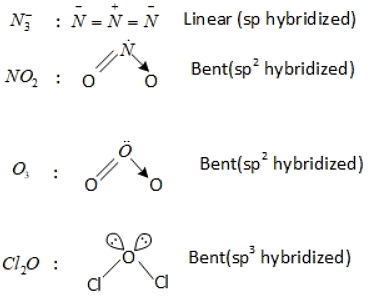
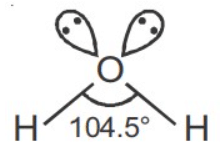
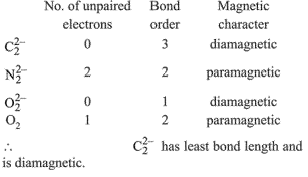
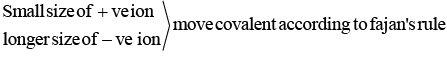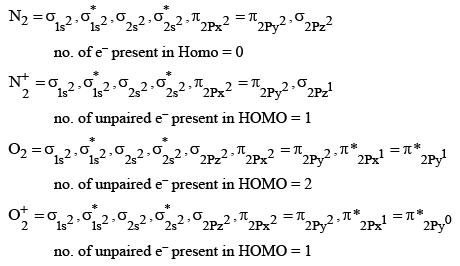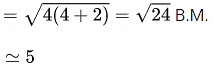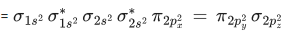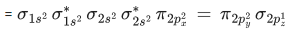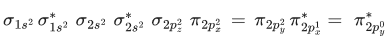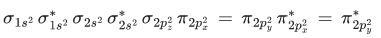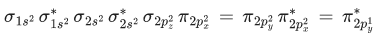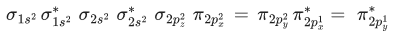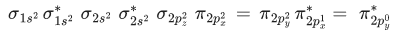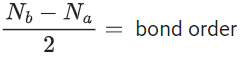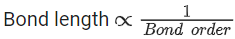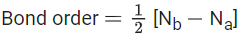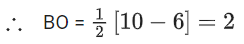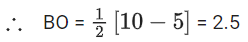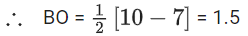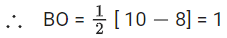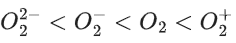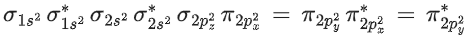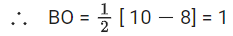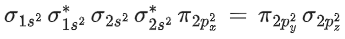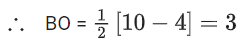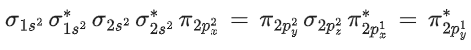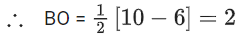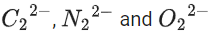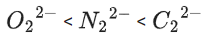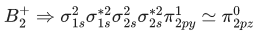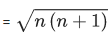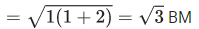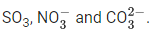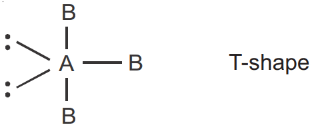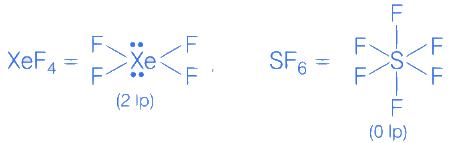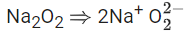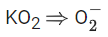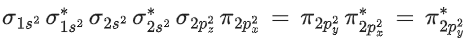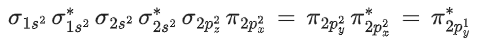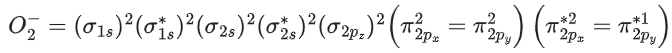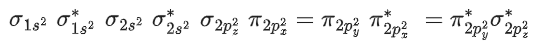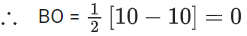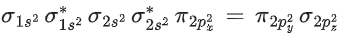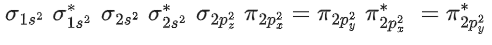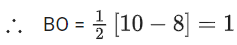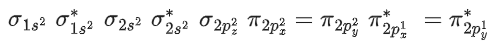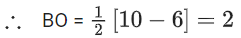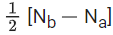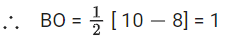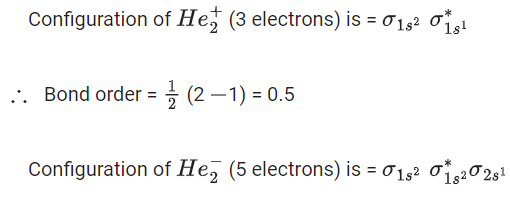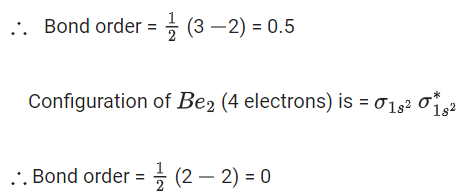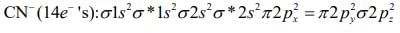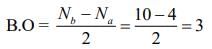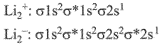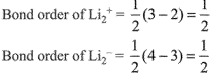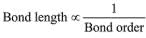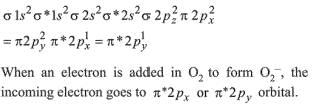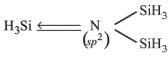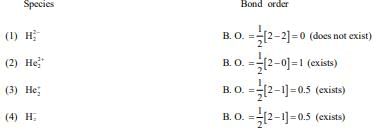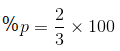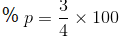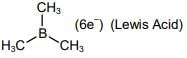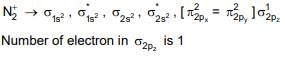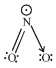Q.1. Order of Covalent bond;
A. KF > KI; LiF > KF
B. KF < KI; LiF > KF
C. SnCl4 > SnCl2; CuCl > NaCl
D. LiF > KF; CuCl < NaCl
E. KF < KI; CuCl > NaCl
Choose the correct answer from the options given below: (JEE Main 2023)
(a) C, E only
(b) B, C, E only
(c) A, B only
(d) B, C only
Ans. b
Q.2. What is the number of unpaired electrons in the highest occupied molecular orbital of the following species : N2; N2+ ; O2; O2+ ? (JEE Main 2023)
(a) 2,1,0,1
(b) 0, 1, 0, 1
(c) 0,1,0,1
(d) 2,1,2,1
Ans. b
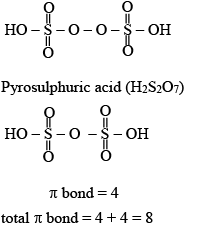
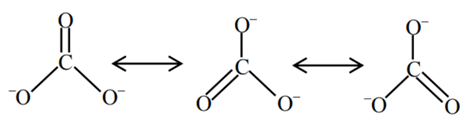
Q.3. Sum of π - bonds present in peroxodisulphuric acid and pyrosulphuric acid is: (JEE Main 2023)
Ans. 8
Peroxodisulphuric acid (H2S2O8)
Q.4. Thermal decomposition of AgNO3 produces two paramagnetic gases. The total number of electrons present in the antibonding molecular orbitals of the gas that has the higher number of unpaired electrons is ______. (JEE Advanced 2022)
Ans. 6
Number of antibonding electron in O2 = 6.
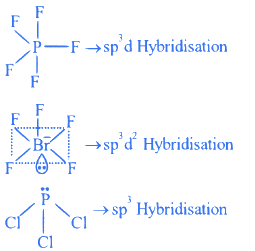
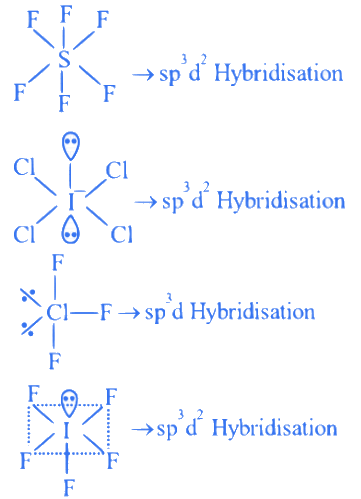
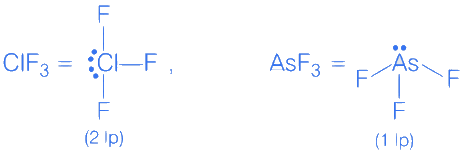
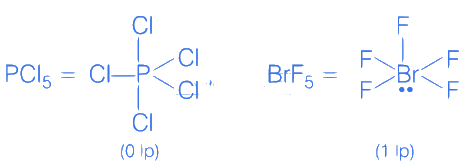
Q.5. Consider, PF5, BrF5, PCl3, SF6,[ICl4]−,ClF3 and IF5.
Amongst the above molecule(s)/ion(s), the number of molecule(s)/ion(s) having sp3 d2 hybridisation is __________. (JEE Main 2022)
Ans. 4
Q.6. The number of interhalogens from the following having square pyramidal structure is :
ClF3, IF7, BrF5, BrF3, I2Cl6, IF5, ClF, ClF5 (JEE Main 2022)
Ans. 3
CIF3 → 3σ bond + 2 lone pair
IF7 → 7σ bond +0 lone pair
BrF5 → 5σ bond +1 lone pair → Square pyramidal
BrF3 → 3σ bond +2 lone pair
I2Cl6 → 4σ bond +2 lone pair
IF5 → 5σ bond +1 lone pair → Square pyramidal
CIF → 1σ bond +3 lone pair
CIF5 → 5σ bond +1 lone pair → Square pyramidal
Q.7. The number of paramagnetic species among the following is ___________. (JEE Main 2022)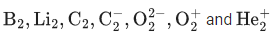
Ans. 4
Those species which have unpaired electrons are called paramagnetic species.
And those species which have no unpaired electrons are called diamagnetic species.
B2 has 10 electrons.
Molecular orbital configuration of B2 isHere two unpaired electrons present. So it is paramagnetic.
has 18 electrons.
Moleculer orbital configuration ofis
Here is no unpaired electron so it is diamagnetic.
has 15 electrons.
Moleculer orbital configuration of O2+ is
Here 1 unpaired electron present, so it is paramagnetic.
C2 has 12 electrons.
Moleculer orbital configuration of C2
Here no unpaired electron present, so it is diamagnetic.
has 13 electrons.
Moleculer orbital configuration ofis
Here 1 unpaired electron present, so it is paramagnetic.
Li2 has 6 electrons.
Here no unpaired electron present, so it is diamagnetic.
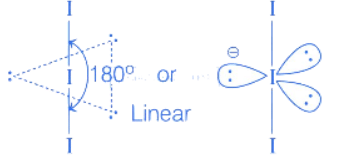
Configuration of(3 electrons) is
Here 1 unpaired electron present, so it is paramagnetic.
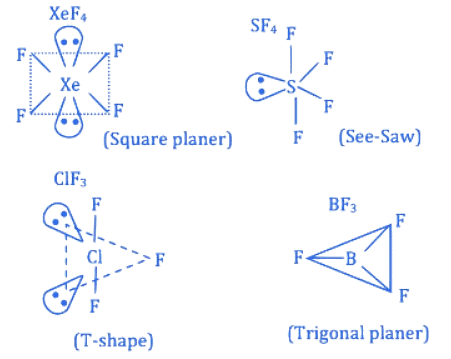
Q.8. The number of molecule(s) or ion(s) from the following having non-planar structure is ____________. (JEE Main 2022)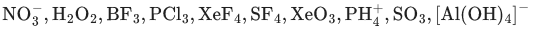
Ans. 6
→ Trigonal planar (Planar)
H2O2 → Open book (Non-planar)
BF3 → Trigonal planar (Planar)
PCl3 → Pyramidal (Non-planar)
XeF4 → Square planar (Planar)
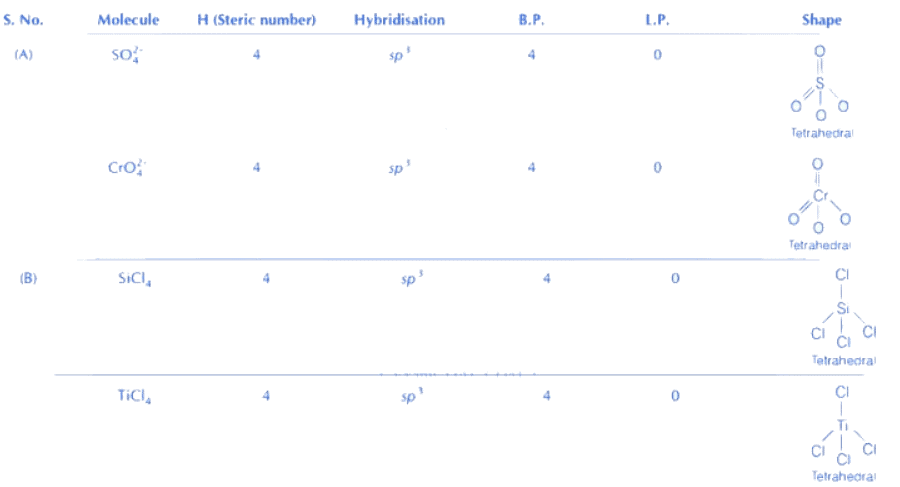
SF4 → See-Saw (Non-planar)
XeO3 → Pyramidal (Non-planar)→ Tetrahedral (Non-planar)
SO3 → Trigonal planar (Planar)
[Al(OH)4]− → Tetrahedral (Non-planar)
Q.9. According to MO theory, number of species/ions from the following having identical bond order is ________. (JEE Main 2022)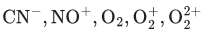
Ans. 3
have bond order of 3
O2 has bond order of 2
O2+ has bond order of 2.5
∴ 3 species have similar bond order.
Q.10. Amongst the following, the number of oxide(s) which are paramagnetic in nature is
Na2O, KO2, NO2, N2O, ClO2, NO, SO2, Cl2O (JEE Main 2022)
Ans. 4
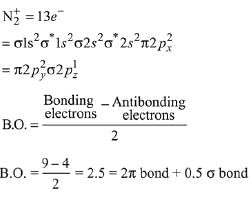
Paramagnetic species: KO2, NO2, ClO2, NO
Diamagnetic species are: Na2O, N2O, SO2, Cl2O
Q.11. The spin-only magnetic moment value of the compound with strongest oxidizing ability among MnF4, MnF3 and MnF2 is ____________ B.M. [nearest integer] (JEE Main 2022)
Ans. 5
MnF3 has the strongest oxidizing abilitySo, spin only magnetic moment
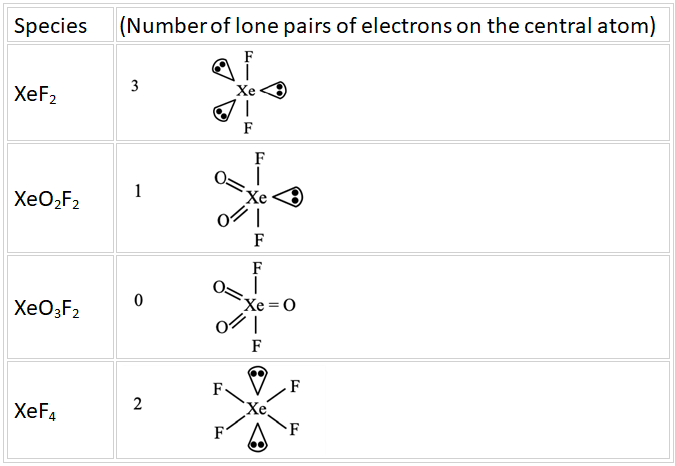
Q.12. The sum of number of lone pairs of electrons present on the central atoms of XeO3, XeOF4 and XeF6, is ______________ (JEE Main 2022)
Ans. 3
From structure, it is clear that it has five bond pairs and one lone pair.
Q.13. Among the following species  the number of species showing diamagnesim is _______________. (JEE Main 2022)
the number of species showing diamagnesim is _______________. (JEE Main 2022)
Ans. 2
Those species which have unpaired electrons are called paramagnetic species.
And those species which have no unpaired electrons are called diamagnetic species.
(1) N2 has 14 electrons.
Moleculer orbital configuration of N2
Here no unpaired electron present, so it is diamagnetic.
(2) Moleculer orbital configuration of N2+ (13 electrons)
Here inunpaired electron present, so it is paramagnetic.
(3)has 16 electrons.
Moleculer orbital configuration ofis
Here 2 unpaired electron present, so it is paramagnetic.
(4)has 15 electrons.
Moleculer orbital configuration ofis
Here 1 unpaired electron present, so it is paramagnetic.
(a)has 18 electrons.
Moleculer orbital configuration ofis
Here is no unpaired electron so it is diamagnetic.
(b)has 17 electrons.
Moleculer orbital configuration ofis
Here 1 unpaired electron present, so it is paramagnetic.
(c) O2 has 16 electrons.
Moleculer orbital configuration of O2 is
Here 2 unpaired electron present, so it is paramagnetic.
(d)has 15 electrons.
Moleculer orbital configuration ofis
Here 1 unpaired electron present, so it is paramagnetic.
Q.14. Amongst the following, the number of molecule/(s) having net resultant dipole moment is ____________.
NF3, BF3, BeF2, CHCl3, H2S, SiF4, CCl4, PF5 (JEE Main 2022)
Ans. 3
Q.15. Amongst BeF2, BF3, H2O, NH3, CCl4 and HCl, the number of molecules with non-zero net dipole moment is ____________. (JEE Main 2022)
Ans. 3
Q.16. Amongst SF4, XeF4, CF4 and H2O, the number of species with two lone pairs of electrons is _____________. (JEE Main 2022)
Ans. 2
Q.17. The hybridization of P exhibited in PF5 is spxdy. The value of y is __________. (JEE Main 2022)
Ans. 1
Q.18. Number of lone pairs of electrons in the central atom of SCl2, O3, ClF3 and SF6, respectively, are: (JEE Main 2022)
(a) 0, 1, 2 and 2
(b) 2, 1, 2 and 0
(c) 1, 2, 2 and 0
(d) 2, 1, 0 and 2
Ans. b
The number of lone pair of electrons in the central atom of SCl2, O3, ClF3 and SF6 are 2, 1, 2 and 0 respectively
Their structures are as,
Q.19. Which of the following pair of molecules contains odd electron molecule and an expanded octet molecule? (JEE Main 2022)
(a) BCl3 and SF6
(b) NO and H2SO4
(c) SF6 and H2SO4
(d) BCl3 and NO
Ans. b
(A) BCl3 - Even electron molecules
SF6 - Expended octet molecules
(B) NO - Odd electron molecules
H2SO4 - Expanded octet
(C) SF6 − Even electron molecules
H2SO4 - Expanded octet
(D) BCl3 − Even electron molecules
NO - odd electron molecules
Q.20. Match List - I with List - II: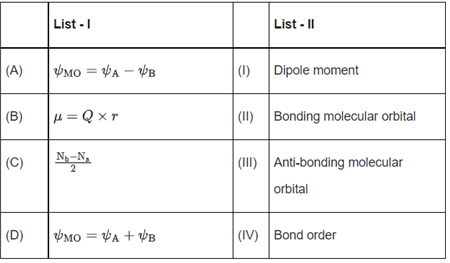 Choose the correct answer from the options given below: (JEE Main 2022)
Choose the correct answer from the options given below: (JEE Main 2022)
(a) (A)−(II), (B)−(I), (C)−(IV), (D)−(III)
(b) (A)−(III), (B)−(IV), (C)−(I), (D)−(II)
(c) (A)−(III), (B)−(I), (C)−(IV), (D)−(II)
(d) (A)−(III), (B)−(IV), (C)−(II), (D)−(I)
Ans. c
ΨA − ΨB = ΨMO is anti-boding molecular orbital
μ = Q × r is dipole moment
ΨA + ΨB = ΨMO is boding molecular orbital.
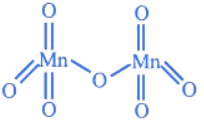
Q.21. The total number of Mn = O bonds in Mn2O7 is __________. (JEE Main 2022)
(a) 4
(b) 5
(c) 6
(d) 3
Ans. c
Structure of Mn2O7 is as:
∴ There are total 6M = O bonds are present in Mn2O7 compound.
Q.22. Given below are two statements.
Statement I: O2, Cu2+, and Fe3+ are weakly attracted by magnetic field and are magnetized in the same direction as a magnetic field.
Statement II: NaCl and H2O are weakly magnetized in opposite direction to magnetic field.
In the light of the above statements, choose the most appropriate answer from the options given below. (JEE Main 2022)
(a) Both Statement I and Statement II are correct.
(b) Both Statement I and Statement II are incorrect.
(c) Statement I is correct but Statement II is incorrect.
(d) Statement I is incorrect but Statement II is correct.
Ans. a
O2, Cu2+ and Fe3+ have 2, 1 and 5 unpaired electrons respectively, so these are the paramagnetic species. Hence, they are attracted by magnetic field.
NaCl and H2O are the diamagnetic species so they are repelled by the magnetic field.
Q.23. Arrange the following in increasing order of their covalent character.
A. CaF2
B. CaCl2
C. CaBr2
D. CaI2
Choose the correct answer from the options given below. (JEE Main 2022)
(a) B < A < C < D
(b) A < B < C < D
(c) A < B < D < C
(d) A < C < B < D
Ans. b
From Fajan's rule, for a given metal ion, as the size of anion increases, polarizability of anion increases and hence covalent character of the given ionic compound increases.
Hence, the increasing order of covalent character is CaF2 < CaCl2 < CaBr2 < Cal2.
Q.24. Match List-I with List-II: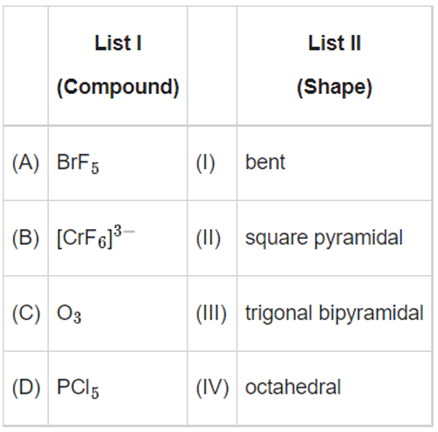 Choose the correct answer from the options given below: (JEE Main 2022)
Choose the correct answer from the options given below: (JEE Main 2022)
(a) (A)−(I), (B)−(II), (C)−(III), (D)−(IV)
(c) (A)−(IV), (B)−(III), (C)−(II), (D)−(I)
(c) (A)−(II), (B)−(IV), (C)−(I), (D)−(III)
(d) (A)−(III), (B)−(IV), (C)−(II), (D)−(I)
Ans. c
(A) BrF5 − square pyramidal
(B) [CrF6]3− − octahedral
(C) O3 − bent
(D) PCl5 - trigonal bipyramidal
Q.25. Match List I with List II: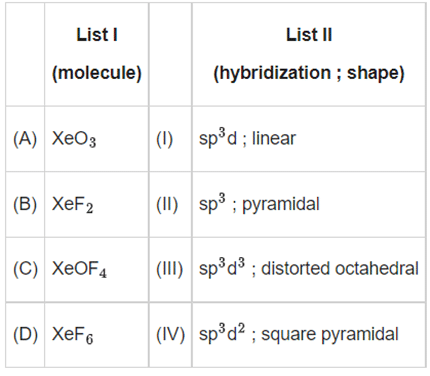 Choose the correct answer from the options given below: (JEE Main 2022)
Choose the correct answer from the options given below: (JEE Main 2022)
(a) A-II, B-I, C-IV, D-III
(b) A-II, B-IV, C-III, D-I
(c) A-IV, B-II, C-III, D-I
(d) A-IV, B-II, C-I, D-III
Ans. a
XeO3 − sp3, Pyramidal
XeF2 − sp3d, linear
XeOF4 − sp3d2, Square Pyramidal
XeF6 − sp3d3, distorted octahedral
Q.26. Number of lone pair(s) of electrons on central atom and the shape BrF3 molecule respectively, are (JEE Main 2022)
(a) 0, triangular planar.
(b) 1, pyramidal.
(c) 2, bent T-shape.
(d) 1, bent T-shape.
Ans. c
Q.27. Consider the species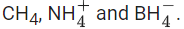 Choose the correct option with respect to the these species. (JEE Main 2022)
Choose the correct option with respect to the these species. (JEE Main 2022)
(a) They are isoelectronic and only two have tetrahedral structures.
(b) They are isoelectronic and all have tetrahedral structures.
(c) Only two are isoelectronic and all have tetrahedral structures.
(d) Only two are isoelectronic and only two have tetrahedral structures.
Ans. b
Q.28. In the structure of SF4, the lone pair of electrons on S is in. (JEE Main 2022)
(a) equatorial position and there are two lone pair-bond pair repulsions at 90∘.
(b) equatorial position and there are three lone pair-bond pair repulsions at 90∘.
(c) axial position and there are three lone pair-bond pair repulsion at 90∘.
(d) axial position and there are two lone pair-bond pair repulsion at 90∘.
Ans. a
SF4 → sp3d hybridisation.The lone pair of electrons on S is in an equatorial position and there are two lone pair-bond pair repulsions at 90°.
Q.29. The correct order of increasing intermolecular hydrogen bond strength is (JEE Main 2022)
(a) HCN < H2O < NH3
(b) HCN < CH4 < NH3
(c) CH4 < HCN < NH3
(d) CH4 < NH3 < HCN
Ans. c
Due to the high difference in electronegativity of H and N the H-bond strength of NH3 is highest. There is no H-bond in CH4.
CH4 < HCN < NH3
Q.30. Identify the incorrect statement for PCl5 from the following. (JEE Main 2022)
(a) In this molecule, orbitals of phosphorous are assumed to undergo sp3d hybridization.
(b) The geometry of PCl5 is trigonal bipyramidal.
(c) PCl5 has two axial bonds stronger than three equatorial bonds.
(d) The three equatorial bonds of PCl5 lie in a plane.
Ans. c
PCl5All three equatorial bonds in a plane
sp3d hybridization
Trigonal bipyramidal
Axial bonds are weaker than equatorial bonds.
Q.31. Based upon VSEPR theory, match the shape (geometry) of the molecules in List-I with the molecules in List-II and select the most appropriate option. (JEE Main 2022)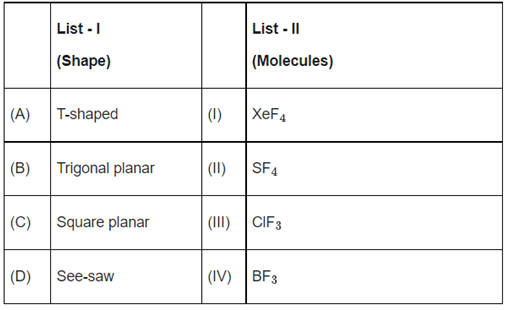 (a) (A) - (I), (B) - (II), (C) - (III), (D) - (IV)
(a) (A) - (I), (B) - (II), (C) - (III), (D) - (IV)
(b) (A) - (III), (B) - (IV), (C) - (I), (D) - (II)
(c) (A) - (III), (B) - (IV), (C) - (I), (D) - (I)
(d) (A) - (IV), (B) - (III), (C) - (I), (D) - (II)
Ans. b
Q.32. Consider the ions/molecule 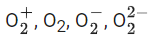 For increasing bond order the correct option is: (JEE Main 2022)
For increasing bond order the correct option is: (JEE Main 2022)
(a)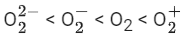
(b)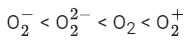
(c)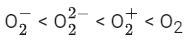
(d)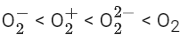
Ans. a
Note:
(1) Bond strength ∝ Bond order
(2)
(3)
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
(4) upto 14 electrons, molecular orbital configuration isHere Na = Anti bonding electron = 4 and Nb = 10
(5) After 14 electrons to 20 electrons molecular orbital configuration is - - -Here Na = 10
and Nb = 10
In O atom 8 electrons present, so in O2, 8 × 2 = 16 electrons present.
Then inno of electrons = 15
inno of electrons = 17
inno of electrons = 18
∴ Molecular orbital configuration of O2 (16 electrons) is∴ Na = 6
Nb = 10
Molecular orbital configuration of(15 electrons) is
∴ Nb = 10
Na = 5
Molecular orbital configuration of(17 electrons) is
∴ Nb = 10
Na = 7
Molecular orbital configuration of(18 electrons) is
∴ Nb = 10
Na = 8
So, correct order of Bond order is
Q.33. Number of electron deficient molecules among the following: (JEE Main 2022)
PH3, B2H6, CCl4, NH3, LiH and BCl3 is
(a) 0
(b) 1
(c) 2
(d) 3
Ans. c
Electron deficient species have less than 8 electrons (or two electrons for H ) in their valence (incomplete octet).
B2H6, BCl3 have incomplete octet.
Q.34. Bonding in which of the following diatomic molecule(s) become(s) stronger, on the basis of MO Theory, by removal of an electron?
(A) NO
(B) N2
(C) O2
(D) C2
(E) B2
Choose the most appropriate answer from the options given below: (JEE Main 2022)
(a) (A), (B), (C) only
(b) (B), (C), (E) only
(c) (A), (C) only
(d) (D) only
Ans. c
If an electron is removed from the anti-bonding orbital, then it will tend to increase the bond order. The HOMO in NO and O2 is antibonding molecular orbital .
Hence, in NO and O2 bond order will increase on loss of electron.
Q.35. The correct order of bond orders of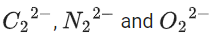 is, respectively (JEE Main 2022)
is, respectively (JEE Main 2022)
(a)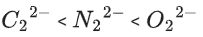
(b)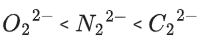
(c)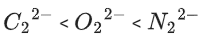
(d)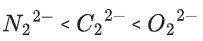
Ans. b
Note:
(1)
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
(2) upto 14 electrons, molecular orbital configuration isHere Na = Anti bonding electron = 4 and Nb = 10
(5) After 14 electrons to 20 electrons molecular orbital configuration is - - -Here Na = 10
and Nb = 10
In O atom 8 electrons present, so in O2, 8 × 2 = 16 electrons present.
inno of electrons = 18
(A) Molecular orbital configuration of(18 electrons) is
∴ Nb = 10
Na = 8
(B)has 14 electrons.
Moleculer orbital configuration ofis
∴ Na = 4
Nb = 10
(C)has 16 electrons.
Moleculer orbital configuration ofis
∴ Na = 6
Nb = 10
The correct order of bond orders of
Q.36. Identify the correct statement for B2H6 from those given below:
(A) In B2H6, all B-H bonds are equivalent.
(B) In B2H6, there are four 3-centre-2-electron bonds.
(C) B2H6 is a Lewis acid.
(D) B2H6 can be synthesized from both BF3 and NaBH4.
(E) B2H6 is a planar molecule.
Choose the most appropriate answer from the options given below: (JEE Main 2022)
(a) (A) and (E) only
(b) (B), (C) and (E) only
(c) (C) and (D) only
(d) (C) and (E) only
Ans. c
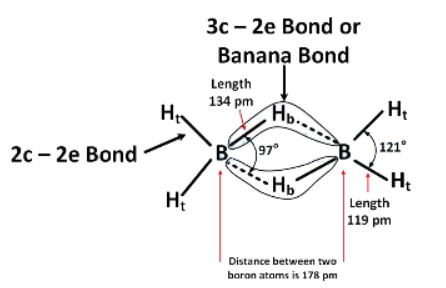
It has two 3-centre-2-electron bonds and four 2-centre-2-electron bonds.
Hence, all B–H bonds are not equivalent.
It is an electron deficient compound as the octet of boron is incomplete.
Hence, it can behave as a Lewis acid.
It can be synthesized from both BF3 and NaBH4
It is a non-planar molecule.Hence, only Statements (C) and (D) are correct.
Q.37. The spin-only magnetic moment value of 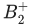 species is _____________ × 10−2 BM. (Nearest integer) [Given : √3 = 1.73] (JEE Main 2021)
species is _____________ × 10−2 BM. (Nearest integer) [Given : √3 = 1.73] (JEE Main 2021)
Ans. 173
It has one unpaired electron.
Spin - only magnetic moment = μ
n = Number of unpaired electrons
= 1.73 BM
= 1.73 × 10−2 BM
Q.38. According to molecular orbital theory, the number of unpaired electron(s) in 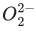 is : (JEE Main 2021)
is : (JEE Main 2021)
Ans. 0
Molecular orbital configuration ofis
Zero unpaired electron
Q.39. The number of hydrogen bonded water molecule(s) associated with stoichiometry CuSO4.5H2O is ____________. (JEE Main 2021)
Ans. 1
One hydrogen bonded H2O molecule
Q.40. The number of species having non-pyramidal shape among the following is ___________. (JEE Main 2021)
(a) SO3
(b) 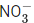
(c) PCl3
(d) 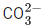
Ans. c
Hence, non-pyramidal species are
Q.41. AB3 is an interhalogen T-shaped molecule. The number of lone pairs of electrons on A is __________. (Integer answer) (JEE Main 2021)
Ans. 2
T-shaped molecule means 3 sigma bond and 2 lone pairs of electron on central atom.
Q.42. The total number of electrons in all bonding molecular orbitals of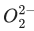 is _________.
is _________.
(Round off to the nearest integer) (JEE Main 2021)
Ans. 10
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
(1) upto 14 electrons, molecular orbital configuration isHere Na = Anti bonding electron = 4 and Nb = 10
(2) After 14 electrons to 20 electrons molecular orbital configuration is - - -
Here Na = 10
and Nb = 10
In O atom 8 electrons present, so in O2, 8 × 2 = 16 electrons present.
Then inno of electrons = 15
inno of electrons = 17
inno of electrons = 18
Molecular orbital configuration of(18 electrons) is
∴ Nb = 10
and Na = 8
Q.43. The difference between bond orders of CO and NO⊕ is x/2 where x = _____________. (Round off to the Nearest Integer) (JEE Main 2021)
Ans. 0
Bond order of CO = 3
Bond order of NO+ = 3
Difference = 0 = x/2
⇒ x = 0
Q.44. The number of lone pairs of electrons on the central I atom in 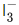 is ____________. (JEE Main 2021)
is ____________. (JEE Main 2021)
Ans. 3
Shape ofis :
The number of lone pairs of electron on the central atom is 3.
Q.45. The number of species below that have two lone pairs of electrons in their central atom is _________. (Round off to the Nearest Integer). (JEE Main 2021)
SF4, BF4−, ClF3, AsF3, PCl5, BrF5, XeF4, SF6
Ans. 2
Q.46. AX is a covalent diatomic molecule where A and X are second row elements of periodic table. Based on Molecular orbital theory, the bond order of AX is 2.5. The total number of electrons in AX is __________. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 15
The compound AX is NO its bond order is 2.5 and it has total 15 electrons.
Note: Total number of electrons equal to 13 will also have the 2.5 bond order. But in this case neutral diatomic molecule will not be possible.
Q.47. Among the following, the number of halide(s) which is/are inert to hydrolysis is _________. (JEE Main 2021)
(a) BF3
(b) SiCl4
(c) PCl5
(d) SF6
Ans. a
BF3 – Shows Partial hydrolysis
SiCl4 – Undergoes hydrolysis readily
PCl5 – Undergoes hydrolysis by addition– elimination mechanism.
SF6 – Due to crowding Inert towards hydrolysis.
Q.48. Number of paramagnetic oxides among the following given oxides is ____________.
Li2O, CaO, Na2O2, KO2, MgO and K2O (JEE Main 2021)
(a) 1
(b) 2
(c) 3
(d) 0
Ans. a
Li2O ⇒ 2Li+ O2−
CaO ⇒ Ca2+ O2−
MgO ⇒ Mg2+ O2−
K2O ⇒ 2K+ O2−
O2− ⇒ Complete octet, diamagnetichas 18 electrons.
Moleculer orbital configuration ofis
Here is no unpaired electron so it is diamagnetic.has 17 electrons.
Moleculer orbital configuration ofis
Here is 1 unpaired electron so it is paramagnetic.
Q.49. Match items of List-I with those of List-II: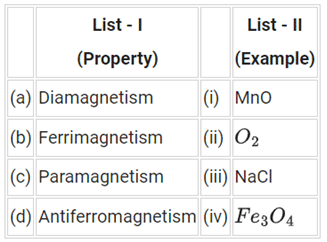 Choose the most appropriate answer from the options given below: (JEE Main 2021)
Choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) (a)-(ii), (b)-(i), (c)-(iii), (d)-(iv)
(b) (a)-(i), (b)-(iii), (c)-(iv), (d)-(ii)
(c) (a)-(iii), (b)-(iv), (c)-(ii), (d)-(i)
(d) (a)-(iv), (b)-(ii), (c)-(i), (d)-(iii)
Ans. c
A. NaCl is diamagnetic because all electrons are paired in Na+ and in Cl− . So, it shows diamagnetism.
B. Fe3O4 is ferrimagnetic because of the presence of the unequal alignment of magnetic moment in opposite direction.
C. O2 molecule has two unpaired electrons. So, it is paramagnetic.
D. In MnO, the orientation of the electrons of Mn and O is such that they cancel their effects, hence it is antiferromagnetic.
Q.50. Match List - I with List - II:  Choose the most appropriate answer from the options given below: (JEE Main 2021)
Choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)
(b) (a)-(iii), (b)-(iv), (c)-(ii), (d)-(i)
(c) (a)-(iii), (b)-(ii), (c)-(iv), (d)-(i)
(d) (a)-(iv), (b)-(ii), (c)-(i), (d)-(iii)
Ans. d
Q.51. The bond order and magnetic behaviour of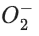 ion are respectively: (JEE Main 2021)
ion are respectively: (JEE Main 2021)
(a) 1.5 and paramagnetic
(b) 1.5 and diamagnetic
(c) 2 and diamagnetic
(d) 1 and paramagnetic
Ans. a
and paramagnetic.
Q.52. Identify the species having one π-bond and maximum number of canonical forms from the following: (JEE Main 2021)
(a) SO3
(b) O2
(c) SO2
(d)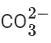
Ans. d
Among SO3, O2, SO2 and, only O2 and
has only one π-bond.
Q.53. In the following the correct bond order sequence is: (JEE Main 2021)
(a)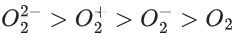
(b)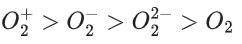
(c)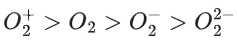
(d)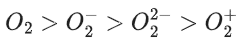
Ans. c
Note:
(1) Bond strength ∝ Bond order
(2)
(3)
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
(4) upto 14 electrons, molecular orbital configuration isHere Na = Anti bonding electron = 4 and Nb = 10
(5) After 14 electrons to 20 electrons molecular orbital configuration is - - -Here Na = 10
and Nb = 10
In O atom 8 electrons present, so in O2, 8 × 2 = 16 electrons present.
Then inno of electrons = 15
inno of electrons = 17
inno of electrons = 18
∴ Molecular orbital configuration of O2 (16 electrons) is
∴ Na = 6
Nb = 10
Molecular orbital configuration of(15 electrons) is
∴ Nb = 10
Na = 5
Molecular orbital configuration of(17 electrons) is
∴ Nb = 10
Na = 7
Molecular orbital configuration of(18 electrons) is
∴ Nb = 10
Na = 8
So, correct order of Bond order is
Q.54. Match List-I with List-II: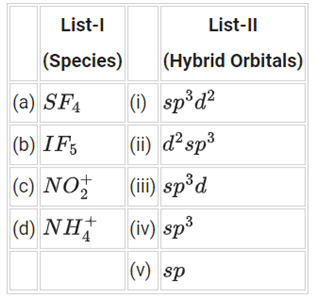 Choose the correct answer from the options given below: (JEE Main 2021)
Choose the correct answer from the options given below: (JEE Main 2021)
(a) (a)-(i), (b)-(ii), (c)-(v) and (d)-(iii)
(b) (a)-(ii), (b)-(i), (c)-(iv) and (d)-(v)
(c) (a)-(iii), (b)-(i), (c)-(v) and (d)-(iv)
(d) (a)-(iv), (b)-(iii), (c)-(ii) and (d)-(v)
Ans. c
(a) SF4 - sp3d hybridization
(b) IF5 - sp3d2 hybridization
(c)- sp hybridization
(d)- sp3 hybridization
Q.55. The hybridisations of the atomic orbitals of nitrogen in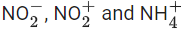 respectively are. (JEE Main 2021)
respectively are. (JEE Main 2021)
(a) sp3, sp2 and sp
(b) sp, sp2 and sp3
(c) sp3, sp and sp2
(d) sp2, sp and sp3
Ans. d
Hybridisation = Number of sigma bonds + number of lone pair of electrons + number of coordinate bond.
Forhybridisation = 2 sigma NO bonds + 1 lone pair on N = 3; so
is sp2-hybridised.
Forhybridisation = 2 sigma NO bonds = 2; so
sp-hybridised.
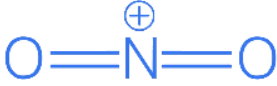
ForHybridisation = 4 sigma NH bonds = 4; so
is sp3-hybridised.
Therefore, hybridisation of N inare sp2, sp, sp3 respectively.
Q.56. The set in which compounds have different nature is: (JEE Main 2021)
(a) B(OH)3 and H3PO3
(b) B(OH)3 and Al(OH)3
(c) NaOH and Ca(OH)2
(d) Be(OH)2 and Al(OH)3
Ans. b
(A) Both are acidic in nature.
B(OH)3 is orthoboric acid. It can accept a pair of electrons from Lewis bases and hence, act as Lewis acid.
H3PO3 is (phosphorus acid) diprotic acid as it readily ionises two protons.(B) B(OH)3 is acidic and Al(OH)3 is amphoteric in nature.
(C) Both NaOH and Ca(OH)2 are basic hydroxides.
(D) Both Be(OH)2 and Al(OH)3 are amphoteric hydroxides.
Hence, only B(OH)3 and Al(OH)3 have different nature.
Q.57. The oxide that shows magnetic property is: (JEE Main 2021)
(a) MgO
(b) SiO2
(c) Mn3O4
(d) Na2O
Ans. c
Mn3O4 is paramagnetic due to presence of unpaired electrons.
Q.58. Amongst the following, the linear species is: (JEE Main 2021)
(a) O3
(b) Cl2O
(c)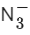
(d) NO2
Ans. c
Q.59. A central atom in a molecule has two lone pairs of electrons and forms three single bonds. The shape of this molecule is: (JEE Main 2021)
(a) trigonal pyramidal
(b) T-shaped
(c) see-saw
(d) planar triangular
Ans. b
sp3d hybridised
T-shaped
Q.60. Given below are two statements: one is labeled as Assertion A and the other is labelled as Reason R:
Assertion A: The H−O−H bond angle in water molecule is 104.5∘.
Reason R: The lone pair - lone pair repulsion of electrons is higher than the bond pair-bond pair repulsion.
In the light of the above statements, choose the correct answer from the options given below: (JEE Main 2021)
(a) Both A and R are true, and R is the correct explanation of A
(b) A is true but R is false
(c) A is false but R is true
(d) Both A and R are true, but R is not the correct explanation of A
Ans. a
due to lp - lp repulsion bond angle decrease.
Q.61. Match List - I with List - II: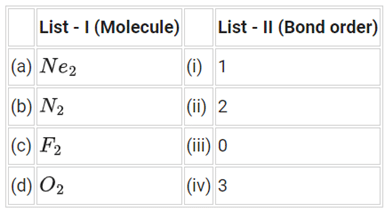 Choose the correct answer from the options given below: (JEE Main 2021)
Choose the correct answer from the options given below: (JEE Main 2021)
(a) (a) → (i), (b) → (ii), (c) → (iii), (d) → (iv)
(b) (a) → (iv), (b) → (iii), (c) → (ii), (d) → (i)
(c) (a) → (iii), (b) → (iv), (c) → (i), (d) → (ii)
(d) (a) → (ii), (b) → (i), (c) → (iv), (d) → (iii)
Ans. c
(1)
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
(2) upto 14 electrons, molecular orbital configuration is
Here Na = Anti bonding electron = 4 and Nb = 10
(5) After 14 electrons to 20 electrons molecular orbital configuration is - - -Here Na = 10
and Nb = 10
(a) Molecular orbital configuration of Ne2 (20 electrons) is
∴ Na = 10
Nb = 10
(Note: All inert gases has BO = 0 and it does not exist as molecule. Here Ne is also an inert gas.)
(b) Moleculer orbital configuration of N2 (14 electrons) is
∴ Na = 4
Nb = 10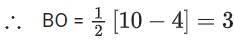
(d) Molecular orbital configuration of F2 (18 electrons) is
∴ Na = 8
Nb = 10
(d) Molecular orbital configuration of O2 (16 electrons) is
∴ Na = 6
Nb = 10
Q.62. Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason R: Fluorine is the most electronegative element and hydrogen bonds in HF are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) Both A and R are true and R is the correct explanation of A
(b) A is true but R is false
(c) A is false but R is true
(d) Both A and R are true but R is NOT the correct explanation of A
Ans. c
Dipole - Dipole are not only the interaction responsible for hydrogen bond formation. Ion-dipole can also be responsible for hydrogen bond formation.
F is most electronegative element and anhydrous HF in solid phase has symmetrical hydrogen bonding.
Q.63. Which among the following species has unequal bond lengths? (JEE Main 2021)
(a) XeF4
(b)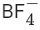
(c) SiF4
(d) SF4
Ans. d
(a) XeF4 is sp3d2 hybridised with 4 bond pairs and 1 lone pair and structure is square planar. Here all the bond lengths are equal.(b)
4 bond pair present so angle is 109o 28' and sp3 hybridised. So structure is regular tetrahedral. Here all the bond lengths are equal.
(c) SiF4 is sp3 hybridisation and regular tetrahedral geometry. Here all the bond lengths are equal.
(d) SF4 is sp3d hybridised with 4 bond pairs and 1 lone pair and its expected trigonal bipyramidal geometry gets distorted due to presence of a lone pair of electrons and it becomes distorted tetrahedral or see-saw with the bond angles equal to < 120o and 179o instead of the expected angles of 120o and 180o respectively. Here axial and equitorial both bonds are presents. And we know axial bonds are longer and weaker.
Q.64. According to molecular orbital theory, the species among the following that does not exist is: (JEE Main 2021)
(a)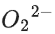
(b)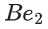
(c)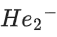
(d)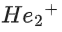
Ans. b
Note:
According to molecules orbital theory, when a molecule have bond order = 0 then that molecule does not exist.
We know, Bond order =
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
(4) upto 14 electrons, molecular orbital configuration isHere Na = Anti bonding electron = 4 and Nb = 10
(5) After 14 electrons to 20 electrons molecular orbital configuration is - - -Here Na = 10
and Nb = 10
Molecular orbital configuration of(18 electrons) is
∴ Nb = 10
Na = 8
Q.65. The correct shape and I−I−I bond angles respectively in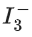 ion are: (JEE Main 2021)
ion are: (JEE Main 2021)
(a) Linear; 180∘
(b) T-shaped; 180∘ and 90∘
(c) Trigonal planar; 120∘
(d) Distorted trigonal planar; 135∘ and 90∘
Ans. a
Hybridisation of central I inis sp3d with 3 lone pair and 2 bond pair.
Shape Linear3 lone pair
Bond angle 180∘ (for linear molecule)
Q.66. The correct set from the following in which both pairs are in correct order of melting point is: (JEE Main 2021)
(a) LiCl > LiF ; NaCl > MgO
(b) LiCl > LiF ; MgO > NaCl
(c) LiF > LiCl ; NaCl > MgO
(d) LiF > LiCl ; MgO > NaCl
Ans. d
Correct option is i.e. LiF > LiCl; MgO > NaCl. Melting point is directly proportional to lattice energy. Lattice energy is the energy required to separate a mole of an ionic solid into gaseous ions. It depends upon charge of ions and size of ions.Both LiF and LiCl having same charge, so melting point will depend on size.
Larger the size of anion, lesser the lattice energy and hence, melting point order is LiF > LiCl.MgO having + 2 charge which is greater than NaCl (+ 1) charge. So, greater the charge on the ions greater will be lattice energy and hence, melting point order is MgO > NaCl.
Q.67. Which of the following are isostructural pairs? (JEE Main 2021)
A.
B. SiCl4, and TiCl4
C. NH3 and NO3-
D. BCl3 and BrCl3
(a) B and C only
(b) C and D only
(c) A and B only
(d) A and C only
Ans. c
Isostructural compounds are those compounds which have same structure as well as same hybridisation.
Formula for find hybridisation: H = (Lone pair + Sigma bond + coordinate bond)
If number of sigma bond (σ), co-ordinate bond and lone pair are same for given pairs, they are isostructural.
Q.68. The bond order and the magnetic characteristic of CN− are (2020)
(a) 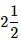 , diamagnetic
, diamagnetic
(b) 3, diamagnetic
(c) 3, paramagnetic
(d) 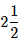 , paramagnetic
, paramagnetic
Ans. b
The molecular electronic configuration of CN– is:
Since, there is no unpaired electron, thus, CN– is diamagnetic in nature.
Q.69. According to molecular orbital theory, which of the following is true with respect to Li2+ and Li2-? (2019)
(a) Li2+ is unstable and Li2- is stable
(b) Li2+ is stable and Li2- is unstable
(c) Both are stable
(d) Both are unstable
Ans. c
Electronic configuratios of Li2+ and Li2-:
Now,
Here, both Li2+ and Li2- have positive bond order, thus both are stable.
Q.70. In which of the following processes, the bond order has increased and paramagnetic character has changed to diamagnetic? (2019)
(a) NO → NO+
(b) N2 → N2+
(c) O2 → O2+
(d) O2 → O22-
Ans. a
In case of NO (paramagnetic) → NO+ (diamagnetic) the bond order has increased from 2.5 to 3.
For other cases:B.O = 2 B.O = 1
Q.71. Two pi and half sigma bonds are present in: (2019)
(a) O2+
(b) N2
(c) O2
(d) N2+
Ans. d
Q.72. Among the following molecules/ions,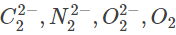 Which one is diamagnetic and has the shortest bond length? (2019)
Which one is diamagnetic and has the shortest bond length? (2019)
(a) O2
(b) 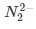
(c) 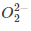
(d) 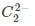
Ans. d
and diamagnetic species has no unpaired electron in their molecular orbitals.
Q.73. Among the following, the molecule expected to be stabilized by anion formation is: (2019)
C2, O2, NO, F2
(a) C2
(b) F2
(c) NO
(d) O2
Ans. a
In case of only C2, incoming electron will enter in the bonding molecular orbital which increases the bond order and stability too. Whereas rest of all takes electron in their antibonding molecular orbital which decreases bond order and stability.
Q.74. Among the following species, the diamagnetic molecule is: (2019)
(a) NO
(b) CO
(c) B2
(d) O2
Ans. b
The molecules with no unpaired electrons are diamagnetic.Since CO has no unpaired electron. Hence, CO is diamagnetic.
Q.75. During the change of O2 to O2- the incoming electron goes to the orbital: (2019)
(a) π2py
(b) σ*2pz
(c) π*2py
(d) π2px
Ans. c
Electronic configuration of O2 is
Q.76. The correct statement among the following is : (2019)
(a) (SiH3)3N is planar and less basic than (CH3)5N.
(b) (SiH3)3N is pyramidal and more basic than (CH3)3N.
(c) (SiH3)3N is pyramidal and less basic than (CH3)3N.
(d) (SiH3)3N is planar and more basic than (CH3)3N.
Ans. a
Due to backbonding of lone pair electrons of nitrogen into vacant d-orbitals of Si, trisilylamine (SiH3)3N is planar. In trimethylamine (CH3)3N, there is no backbonding and hence it is more basic.
Q.77. According to molecular orbital theory, which of the following will not be a viable molecule? (2018)
(a) 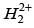
(b) 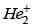
(c) 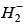
(d) 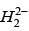
Ans. d
Q.78. In graphite and diamond, the percentage of p-characters of the hybrid orbitals in hybridization are respectively: (2018)
(a) 67 and 75
(b) 33 and 25
(c) 50 and 75
(d) 33 and 75
Ans. a
Graphite
Sp2 hybridization
= 67 %
Diamond
Sp3 hybridisation
= 75%
Q.79. Which of the following is a Lewis acid? (2018)
(a) B(CH3)3
(b) PH3
(c) NaH
(d) NF3
Ans. a
Q.80. In the molecular orbital diagram for the molecular ion, N2+, the number of electrons in the σ2p molecular orbital is: (2018)
(a) 1
(b) 3
(c) 0
(d) 2
Ans. a
Q.81. Which of the following conversions involves change in both shape and hybridisation? (2018)
(a) H2O → H3O+
(b) BF3 → BF4–
(c) CH4 →C2H6
(d) NH3 → NH4+
Ans. b
Q.82. The most polar compound among the following is: (2018)
(a) 
(b) 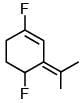
(c) 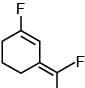
(d) 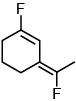
Ans. c
the bond dipole vector of C–F bond is not subtractive.
Q.83. The incorrect geometry is represented by: (2018)
(a) NF3 – trigonal planar
(b) BF3 – trigonal planar
(c) AsF5 – trigonal bipyramidal
(d) H2O – bent
Ans. a
Q.84. Which of the following species is not paramagnetic? (2017)
(a) NO
(b) CO
(c) O2
(d) B2
Ans. b
CO has 14 electrons (even) ∴ It is diamagnetic
NO has 15e-(odd) ∴ It is paramagnetic and has 1 unpaired electron in π*2p molecular orbital.
B2 has 10e- (even) but still paramagnetic and has two unpaired electrons in π2px and π2py (s-p mixing).
O2 has 16 e- (even) but still paramagnetic and has two unpaired electrons in π*2px and π*2py molecular
Q.85. Which of the following is paramagnetic? (2017)
(a) CO
(b) NO+
(c)
(d) B2
Ans. d
No of e– in
CO = 14, NO+ = 14= 18, B2 = 10
According to MOT
B2 is paramagnetic
Q.86. sp3d2 hybridization is not displayed by: (2017)
(a) SF6
(b) BrF5
(c) PF5
(d) [CrF6]3-
Ans. c
SF6 = Sp3d2 BrF5 = SP3d2PF5 = sp3d
Q.87. The group having triangular planar structure is (2017)
(a) BF3, NF3, CO32–
(b) CO32–, NO3–, SO3
(c) NH3, SO3, CO32–
(d) NCl3, BCl3, SO3
Ans. b
CO32–, NO3–, SO3 : SP2 Hybridised
Q.88. The species in which the N atom is in a state of sp hybridization is: (2016)
(a) NO2+
(b) NO2-
(c) NO3-
(d) NO2
Ans. a
The lewis structure of NO2 shows a bent molecular geometry with trigonal planar electron pair geometry hence the hybridization will be sp2
Q.89. Which one of the following statements about water is FALSE? (2016)
(a) Water is oxidized to oxygen during photosynthesis.
(b) Water can act both as an acid and as a base.
(c) There is extensive intramolecular hydrogen bonding in the condensed phase.
(d) Ice formed by heavy water sinks in normal water.
Ans. c
Water possess intermolecular hydrogen bonding in the condensed phase.
FAQs on JEE Main Previous Year Questions (2016- 2025): Chemical Bonding & Molecular Structure
| 1. What is chemical bonding? |  |
| 2. What are the different types of chemical bonds? |  |
| 3. How is the Lewis dot structure used to represent chemical bonding? |  |
| 4. What is hybridization in chemical bonding? |  |
| 5. How do intermolecular forces affect chemical bonding and molecular structure? |  |