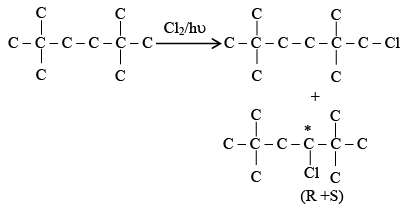
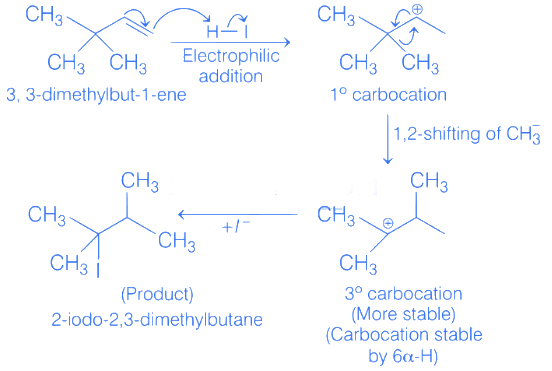
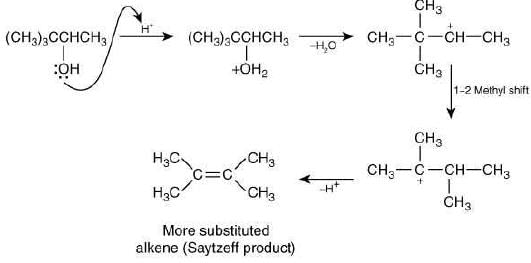
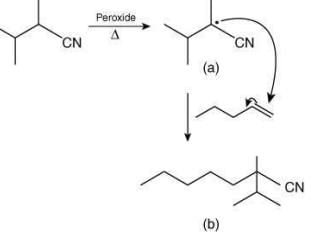
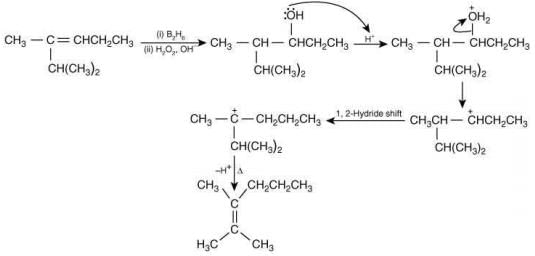
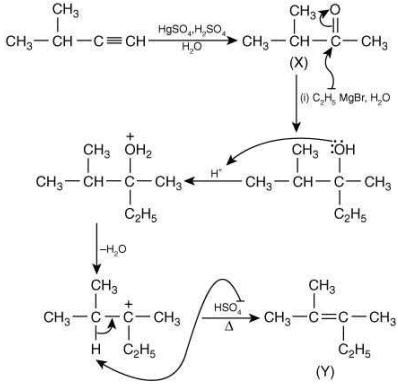
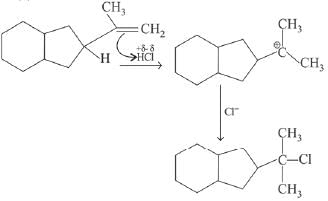
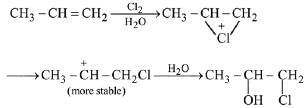
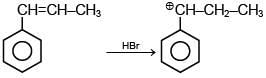
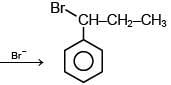
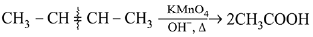Q.1. Maximum number of isomeric monochloro derivatives which can be obtained from 2, 2, 5, 5 tetramethylhexane by chlorination is____________ (JEE Main 2023)
Ans. 3
Q.2. In the following reactions: (JEE Advanced 2022)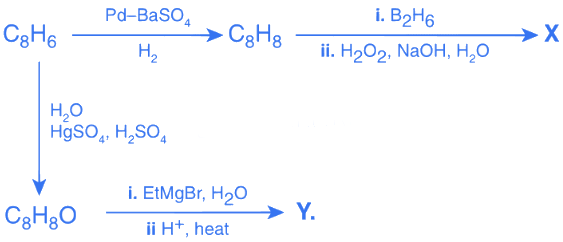 (a)
(a) 
(b) 
(c) 
(d) 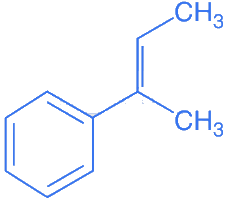
Ans. d
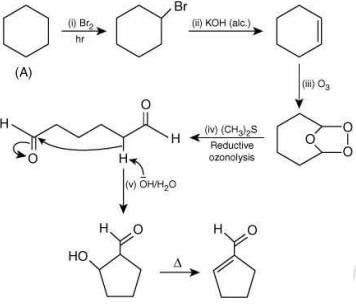
Q.3. The number of −CH2− (methylene) groups in the product formed from the following reaction sequence is _________. (JEE Advanced 2022)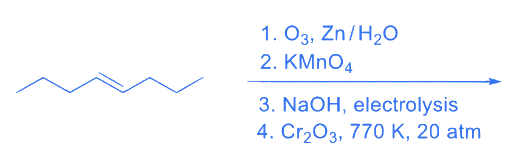
Ans. 0
Q.4. If the reaction sequence given below is carried out with 15 moles of acetylene, the amount of the product D formed (in g ) is ___________. (JEE Advanced 2022)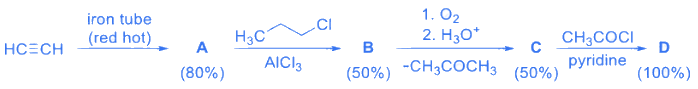 The yields of A,B,C and D are given in parentheses. (JEE Advanced 2022)
The yields of A,B,C and D are given in parentheses. (JEE Advanced 2022)
[Given: Atomic mass of H = 1, C = 12, O = 16, Cl = 35]
Ans. 135.80 and 136.20
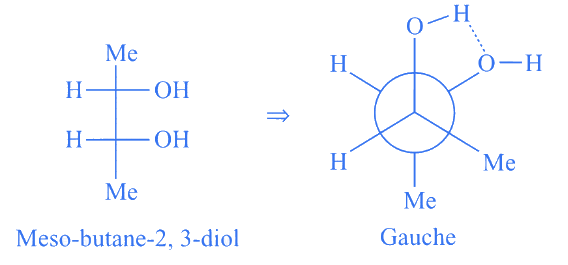
Q.5. Among the following, the conformation that corresponds to the most stable conformation of meso-butane-2,3-diol is (JEE Advanced 2021)
(a) 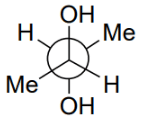
(b) 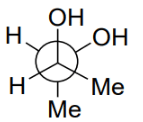
(c) 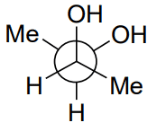
(d) 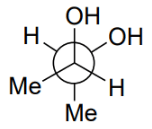
Ans. b
Due to the intramolecular H-bonding, the gauche conformation of butane-2,3-diol is most stable one.
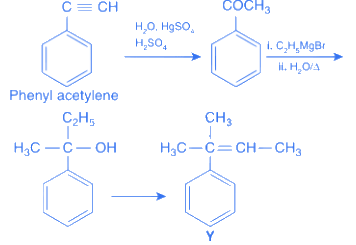
Q.6. A and B in the atmospheric reaction step are : (JEE Main 2022)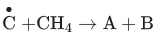
(a) C2H6 and Cl2
(b) 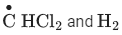
(c)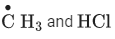
(d) C2H6 and HCl
Ans. c
Q.7. In the given reaction,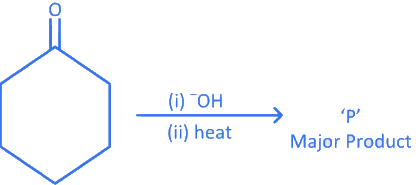 The number of π electrons present in the product 'P' is _________. (JEE Main 2022)
The number of π electrons present in the product 'P' is _________. (JEE Main 2022)
Ans. 4
Q.8.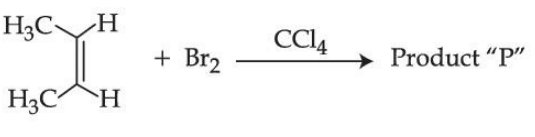 Consider the above chemical reaction. The total number of stereoisomers possible for Product 'P' is _____________. (JEE Main 2021)
Consider the above chemical reaction. The total number of stereoisomers possible for Product 'P' is _____________. (JEE Main 2021)
Ans. 2
The total number of products possible = 2
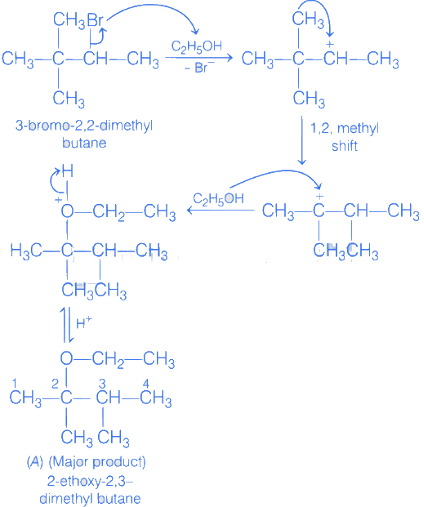
Q.9. In the given reaction 3-Bromo-2, 2-dimethyl butane 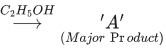 Product A is : (JEE Main 2021)
Product A is : (JEE Main 2021)
(a) 2-Ethoxy-3, 3-dimethyl butane
(b) 1-Ethoxy-3, 3-dimethyl butane
(c) 2-Ethoxy-2, 3-dimethyl butane
(d) 2-Hydroxy-3, 3-dimethyl butane
Ans. c
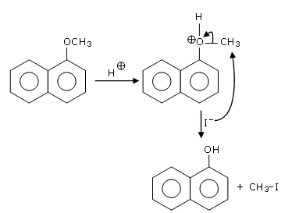
Q.10. Main Products formed during a reaction of 1-methoxy naphthalene with hydroiodic acid are: (JEE Main 2021)
(a) 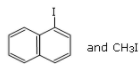
(b) 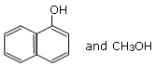
(c) 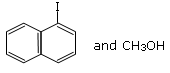
(d) 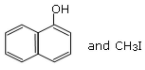
Ans. d
Q.11. Match List - I with List - II : (JEE Main 2021)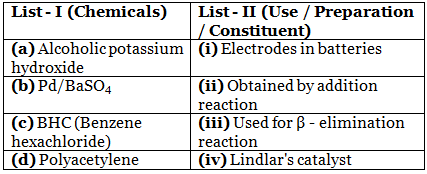 Choose the most appropriate match :
Choose the most appropriate match :
(a) (A) - (ii), (B) - (i), (C) - (iv), (D) - (iii)
(b) (A) - (iii), (B) - (iv), (C) - (ii), (D) - (i)
(c) (A) - (iii), (B) - (i), (C) - (iv), (D) - (ii)
(d) (A) - (ii), (B) - (iv), (C) - (i), (D) - (iii)
Ans. b
Alc. KOH causes elimination
Pd / BaSO4 – Lindlar’s catalyst
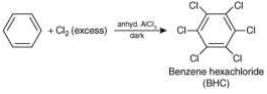
BHC is obtained by the addition reaction of Cl2 with benzene in presence of U.V.
Thin film of polyacetylene can be used as electrode in batteries.
Q.12. The major product of the following reaction is : (JEE Main 2021)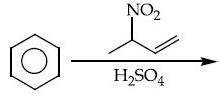 (a)
(a) 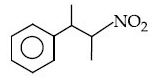
(b) 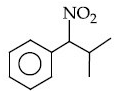
(c) 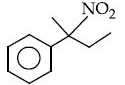
(d) 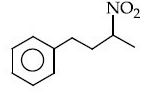
Ans. a
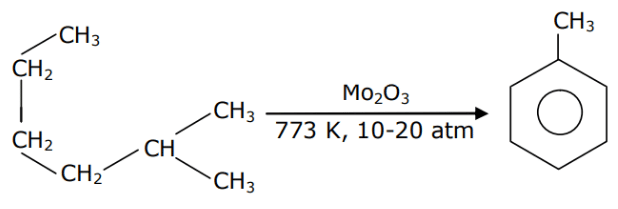
Q.13. Identify A in the given chemical reaction. (JEE Main 2021)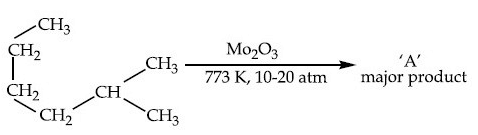 (a)
(a) 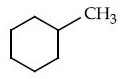
(b) 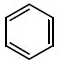
(c) 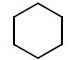
(d) 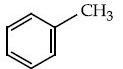
Ans. d
Mo2O3 at 773 K temperature and 10-20-atm pressure is aromatising agent.
Q.14. What is the major product formed by HI on reaction with (JEE Main 2021)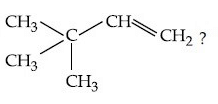
(a) 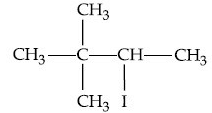
(b) 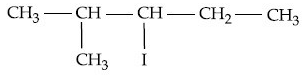
(c) 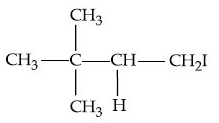
(d) 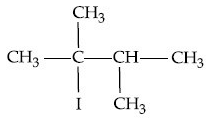
Ans. d
Q.15. Consider the following reactions
(I) (CH3)3CCH(OH)CH3 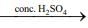
(II) (CH3)3CHCH(Br)CH3 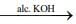
(III) (CH3)2CHCH(Br)CH3 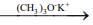
(IV) 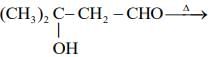
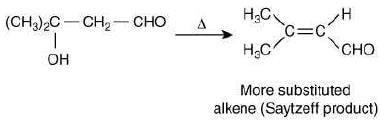
Which of these reaction(s) will not produce Saytzeff product? (2020)
(a) (I), (III) and (IV)
(b) (IV) only
(c) (III) only
(d) (II) and (IV)
Ans. c
The reaction involved is:
(I)
(II)
(III)
(IV)
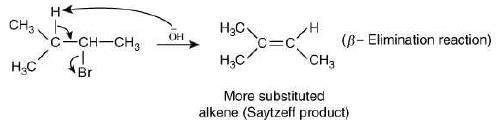
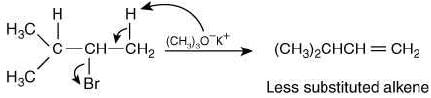
Since, the base used in reaction (III) is bulky, so it will abstract the hydrogen from less hindered carbon atom and will not form Saytzeff alkene.
Q.16. Consider the following reactions:
(I) 
(II) 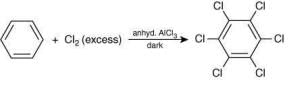
(III) 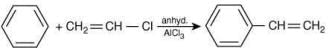
(IV) 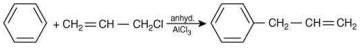
Which of these reactions are possible? (2020)
(a) (I) and (II)
(b) (I) and (IV)
(c) (II), (III) and (IV)
(d) (II) and (IV)
Ans. d
The possible reactions of benzene are:
Reaction (II):
Reaction (IV):
Q.17. The major products A and B in the following reactions are (2020)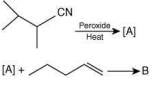
(a) 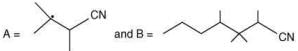
(b) 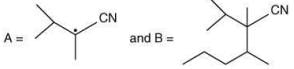
(c) 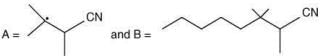
(d) 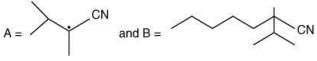
Ans. d
The reaction involved is
Q.18. The major product [B] in the following sequence of reaction is (2020)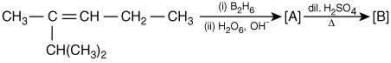 (a)
(a) 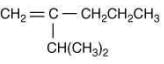
(b) 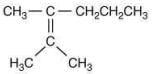
(c) 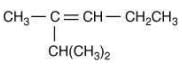
(d) 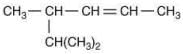
Ans. c
The reaction involved is
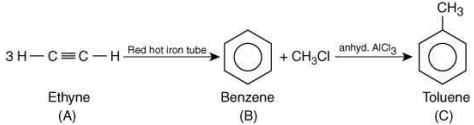
Q.19. In the following sequence of reactions, the maximum number of atoms present in molecule ‘C’ in one plane is _______.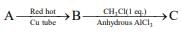
(A is a lowest molecular weight alkyne) (2020)
Ans. 13
The reaction involved is
The molecular formula of toluene is C7H8, thus, the total number of atoms in toluene is 13.
Q.20. The correct order of heat of combustion for following alkadienes is (2020)
(i) 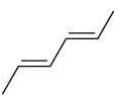
(ii) 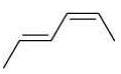
(iii) 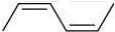 +
+
(a) (I) < (II) < (III)
(b) (I) < (III) < (II)
(c) (III) < (II) < (I)
(d) (II) < (III) < (I)
Ans. a
We know
Heat of combination of alkene ∝ 1/Stability of alkene
Since, trans-alkene is more stable than the cis-alkenes. Hence, the correct order of heat of combustion of the given dienes is: (I) < (II) < (III).
Q.21. The major product (Y) in the following reactions is (2020)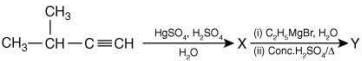 (a)
(a) 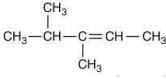
(b) 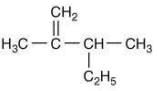
(c) 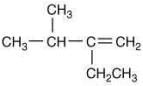
(d) 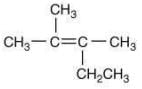
Ans. d
The reaction involved is
Q.22. In the following reaction, A is (2020)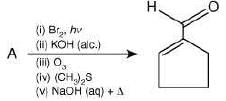 (a)
(a) 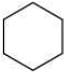
(b) 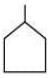
(c) 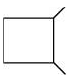
(d) 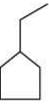
Ans. a
The reaction involved is
Q.23. The number of sp2 hybrid orbitals in a molecule of benzene is (2020)
(a) 24
(b) 6
(c) 18
(d) 12
Ans. c
The number of sp2 hybridized orbitals in benzene is 6 × (1 s-orbital + 2 p-orbitals) = 18 orbitals.
Q.24. The major product obtained in the following conversion is: (2019)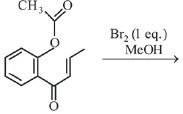 (a)
(a) 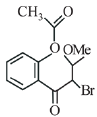
(b) 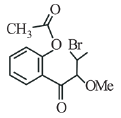
(c) 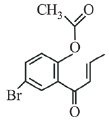
(d) 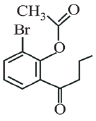
Ans: a
Q.25. The major product of the following reaction is: (2019)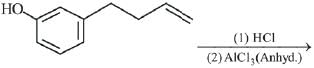 (a)
(a) 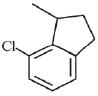
(b) 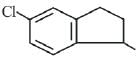
(c) 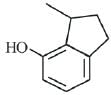
(d) 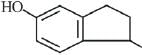
Ans. d
Q.26. The major product of the following reaction is: (2019)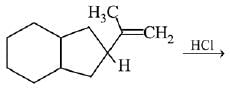
(a) 
(b) 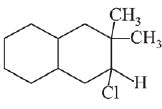
(c) 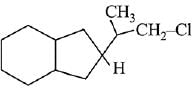
(d) 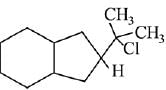
Ans. d
Q.27. The major product of the following reaction is: (2019)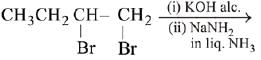 (a) CH3CH = C = CH2
(a) CH3CH = C = CH2
(b) 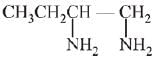
(c) CH3CH = CHCH2NH2
(d) CH3CH2C ≡ CH
Ans. d
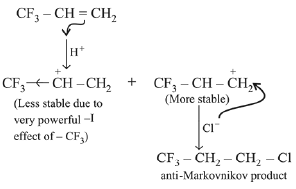
Q.28. Which one of the following alkenes when treated with HCI yields majorly an anti Markovnikov product? (2019)
(a) CH3O — CH = CH2
(b) Cl — CH = CH2
(c) H2N — CH = CH2
(d) F3C — CH = CH2
Ans. d
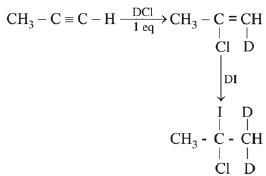
Q.29. The major product of the following reaction is: (2019)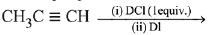 (a) CH3CD(I)CHD(Cl)
(a) CH3CD(I)CHD(Cl)
(b) CH3CD(Cl)CHD(I)
(c) CH3CD2CH(Cl)(I)
(d) CH3C(I)(Cl)CHD2
Ans. d
Both additions follow Markovnikov’s rule.
Q.30. The major product(s) obtained in the following reaction is/are: (2019)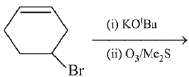 (a)
(a) 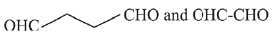
(b) 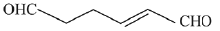
(c) 
(d) 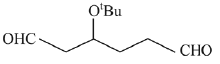
Ans. a
Q.31. The major product of the following addition reaction is (2019)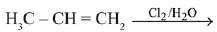 (a)
(a) 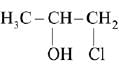
(b) 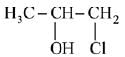
(c) 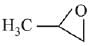
(d) 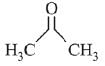
Ans. b
Q.32. But 2-ene on reaction with alkaline KMnO4 at elevated temperature followed by acidification will give: (2019)
(a) 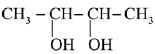
(b) one molecule of CH3CHO and one molecule of CH3COOH
(c) 2 molecules of CH3COOH
(d) 2 molecules of CH3CHO
Ans. c
Q.33. Consider the following reaction: (2019)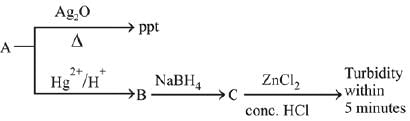 'A' is:
'A' is:
(a) CH ≡ CH
(b) CH3 - C ≡ C - CH3
(c) CH3- C = CH
(d) CH2 = CH2
Ans. c
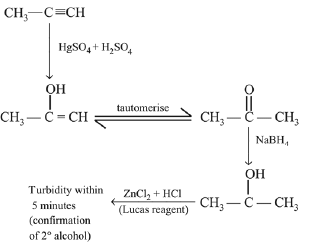
Q.34. The trans-alkenes are formed by the reduction of alkynes with: (2018)
(a) H2 – Pd/C, BaSO4
(b) NaBH4
(c) Na/liq. NH3
(d) Sn-HCl
Ans. (c)
Na/Liq. NH3 reduces alkynes into transalkene (trans addition).
Q.35. The major product of the following reaction is: (2018)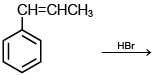 (a)
(a) 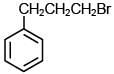
(b) 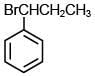
(c) 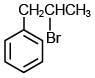
(d) 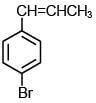
Ans. b
Q.36. Which of the following compounds will most readily be dehydrated to give alkene under acidic condition? (2018)
(a) 4-Hydroxypentan-2-one
(b) 3-Hydroxypentan-2-one
(c) 1-Pentanol
(d) 2-Hydroxycyclopentanone
Ans. a
will most readily be dehydrated to give conjugated alkene.
Q.37. Which of the following, upon treatment with tert-BuONa followed by addition of bromine water, fails to decolourize the colour of bromine? (2017)
(a) 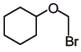
(b) 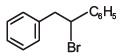
(c) 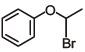
(d) 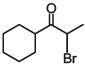
Ans. a
The above product does not have any C = C or C ≡ C bond, so, it will not give Br2-water test.
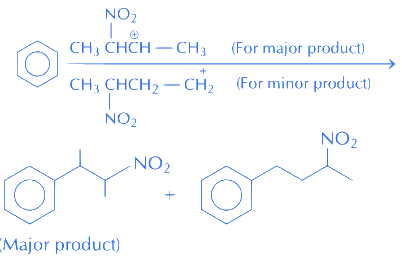
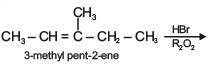
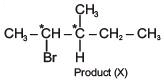
Q.38. 3-Methyl-pent-2-ene on reaction with HBr in presence of peroxide forms an addition product. The number of possible stereoisomers for the product is (2017)
(a) Six
(b) Zero
(c) Two
(d) Four
Ans. d
Since product (X) contains two chiral centres and it is unsymmetrical.
So, its total stereoisomers = 22 = 4.
Q.39. Which of the following compounds will not undergo Friedel Craft's reaction with benzene? (2017)
(a) 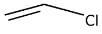
(b) 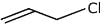
(c) 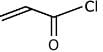
(d) 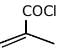
Ans. a
Formation of carbocation is not possible in case of CH2 = CHCl
Q.40. The major product of the following reaction is: (2017)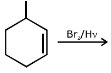 (a)
(a) 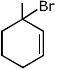
(b) 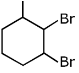
(c) 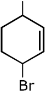
(d) 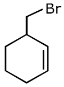
Ans. a
Q.41. The product of the reaction given below is: (2016)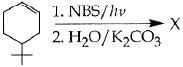 (a)
(a) 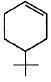
(b) 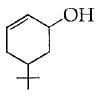
(c) 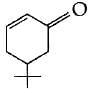
(d) 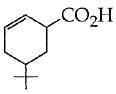
Ans. b
Q.42. Fluorination of an aromatic ring is easily accomplished by treating a diazonium salt with HBF4. Which of the following conditions is correct about this reaction? (2016)
(a) Only heat
(b) NaNO2/Cu
(c) Cu2O/H2O
(d) NaF/Cu
Ans. a
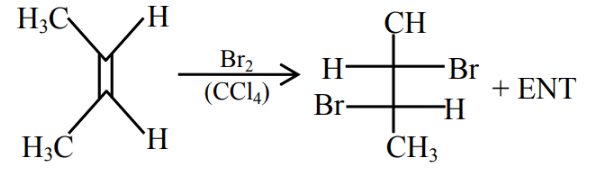
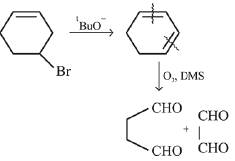
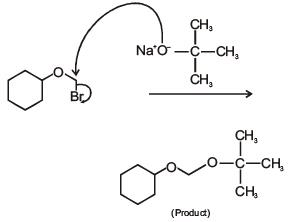
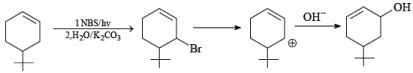
Q.43. Bromination of cyclohexene under conditions given below yields: (2016)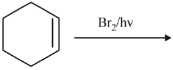 (a)
(a) 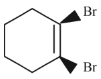
(b) 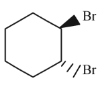
(c) 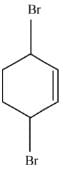
(d) 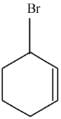
Ans. d
It is free radical substitution reaction.