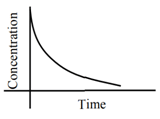
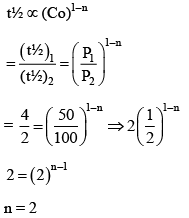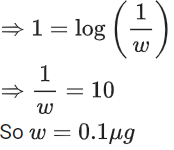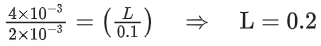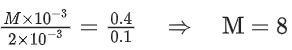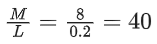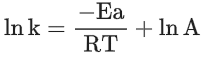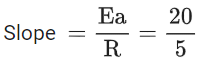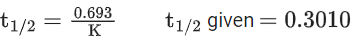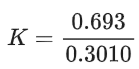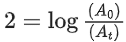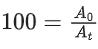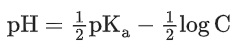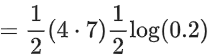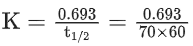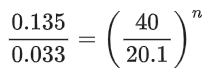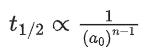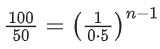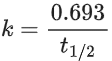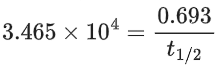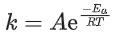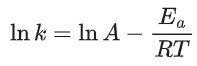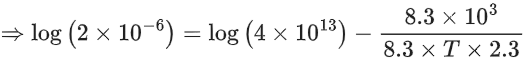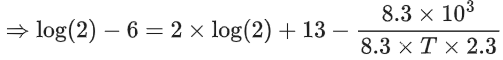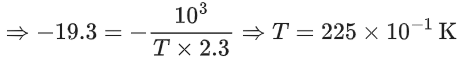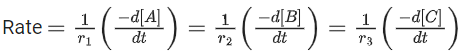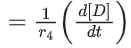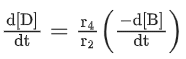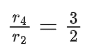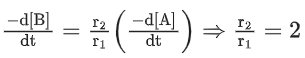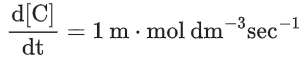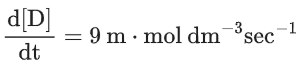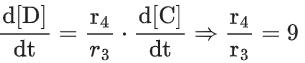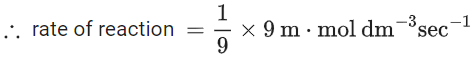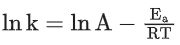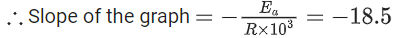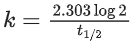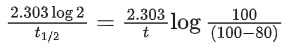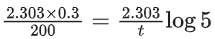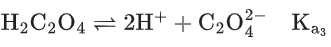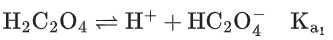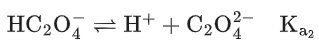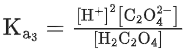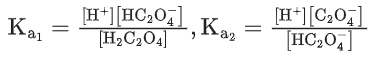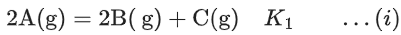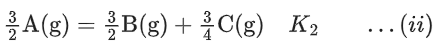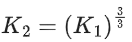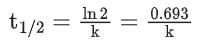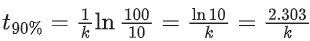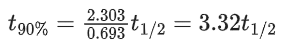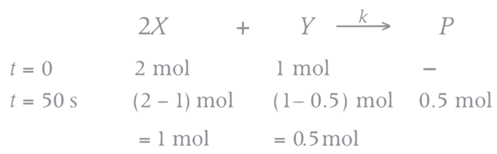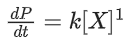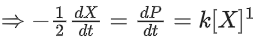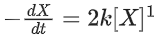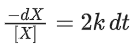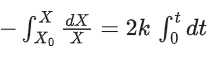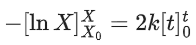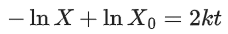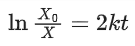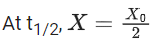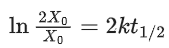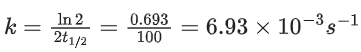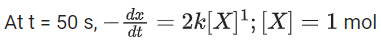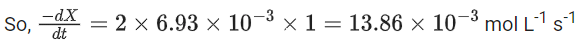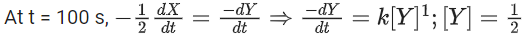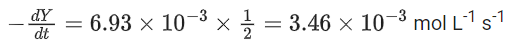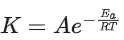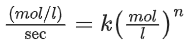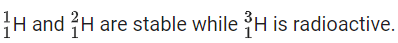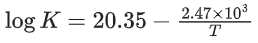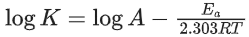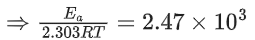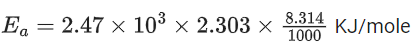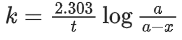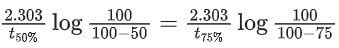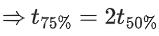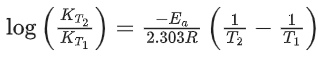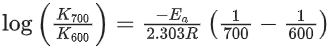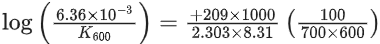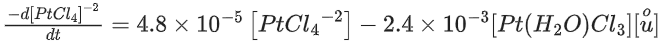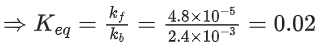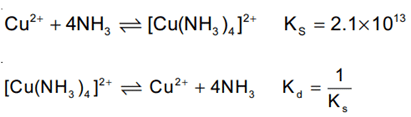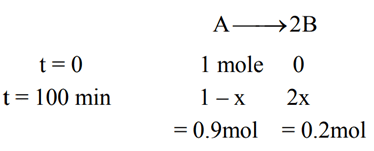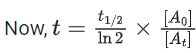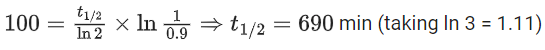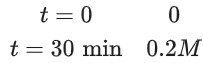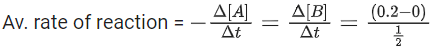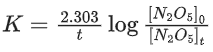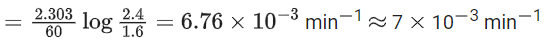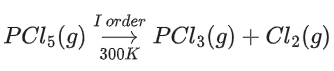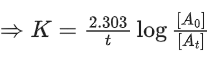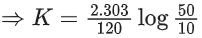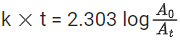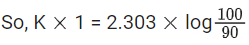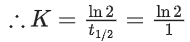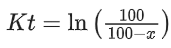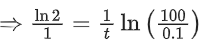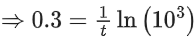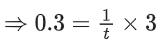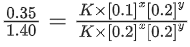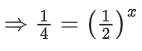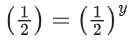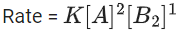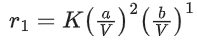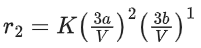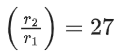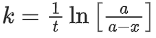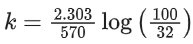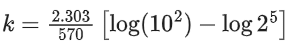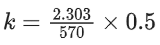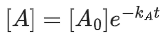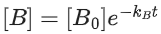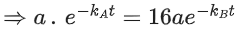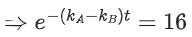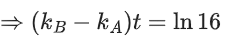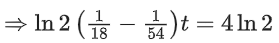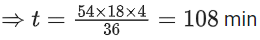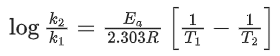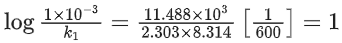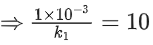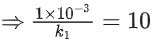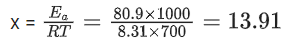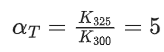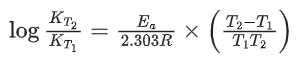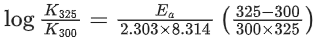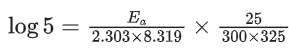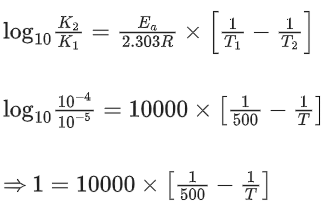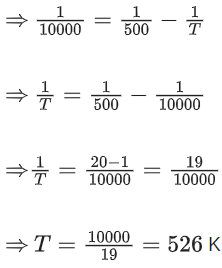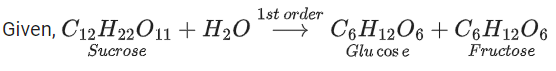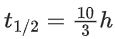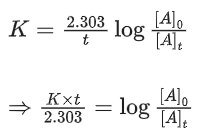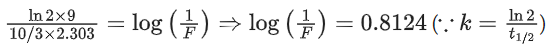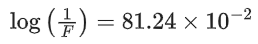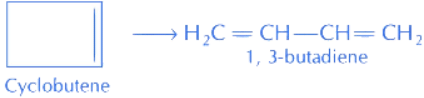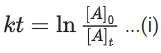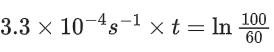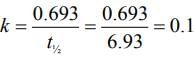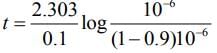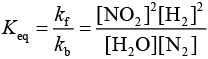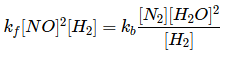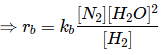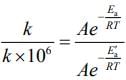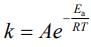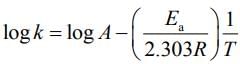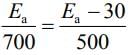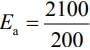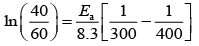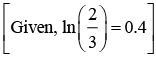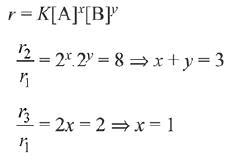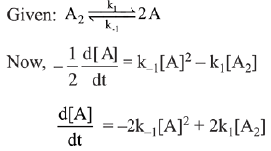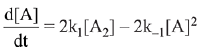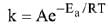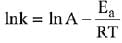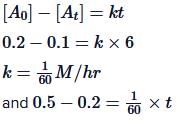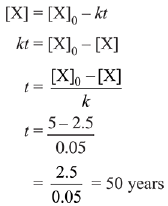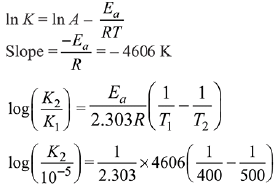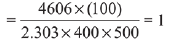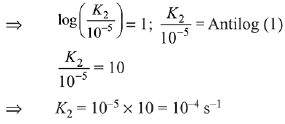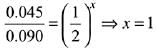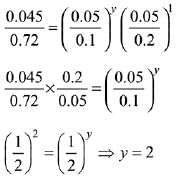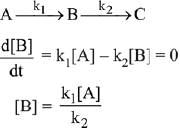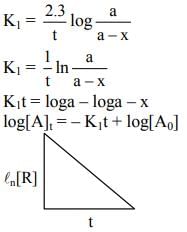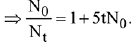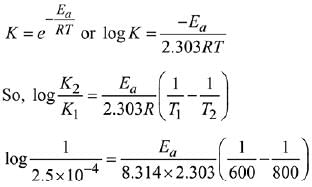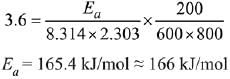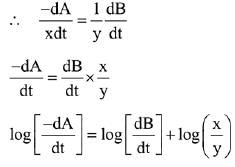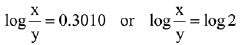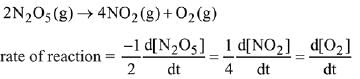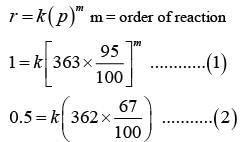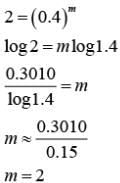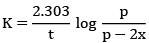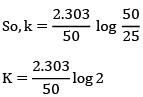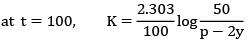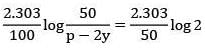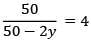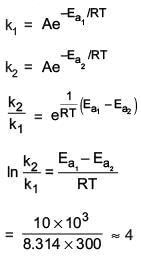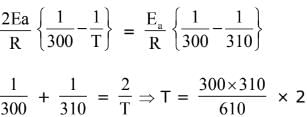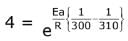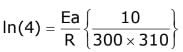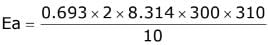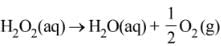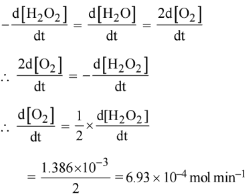Q.1. The number of correct statement/s from the following is (JEE Main 2023)
(a) Larger the activation energy, smaller is the value of the rate constant.
(b) The higher is the activation energy, higher is the value of the temperature coefficient.
(c) At lower temperatures, increase in temperature causes more change in the value of k than at higher temperature
(d) A plot of ln 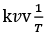 is a straight line with slope equal to
is a straight line with slope equal to 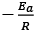
Ans. d
K = Ae–Ea/RT
Here, Ea ↑ K↓
lnK = lnA –Ea/RT
slope of lnK vs 1/T = -Ea/R
The higher is the activation energy, higher is the value of the temperature coefficient.
Q.2. A student has studied the decomposition of a gas AB3 at 25∘C. He obtained the following data. (JEE Main 2023)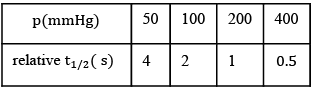 The order of the reaction is
The order of the reaction is
(a) 0 (zero)
(b) 0.5
(c) 1
(d) 2
Ans. d
Q.3. Match the rate expressions in LIST-I for the decomposition of X with the corresponding profiles provided in LIST-II. Xs and k are constants having appropriate units. (JEE Advanced 2022)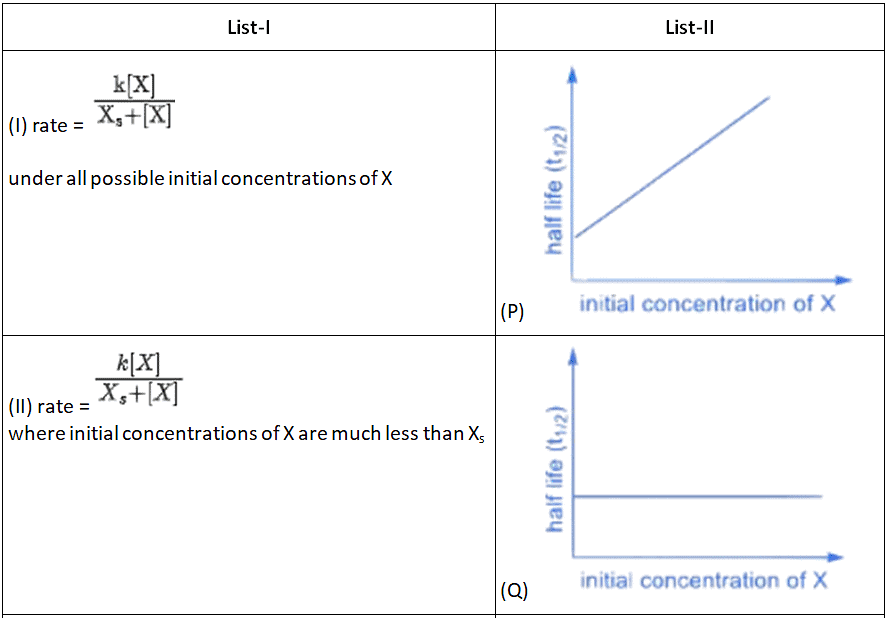
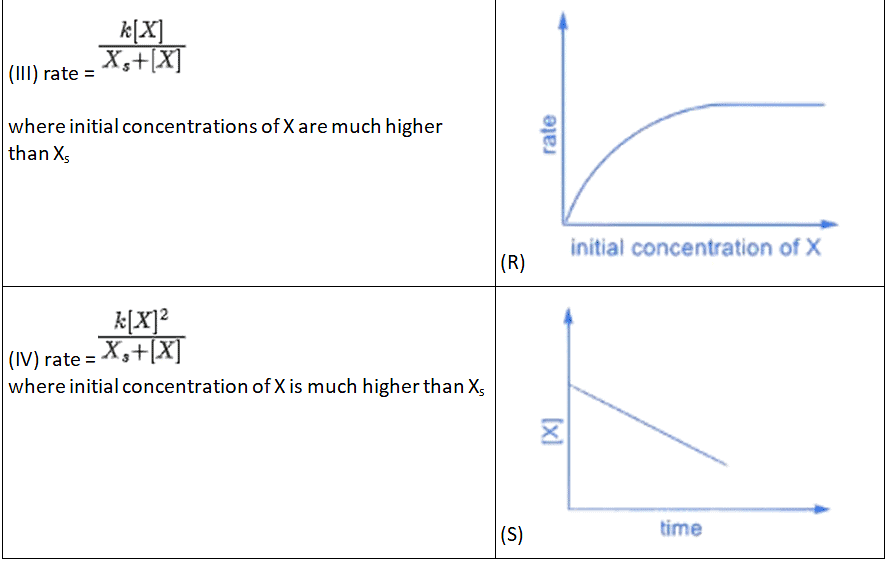
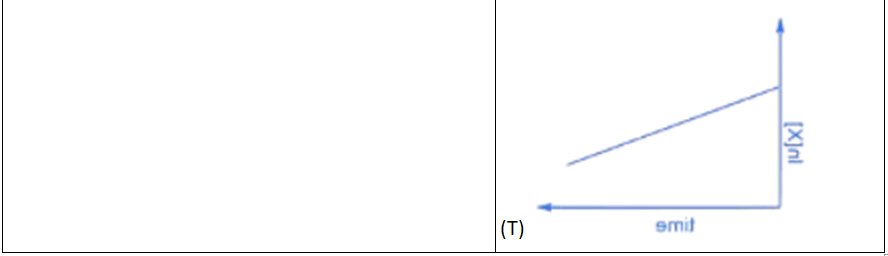 (a) I → P; II → Q; III → S; IV →T
(a) I → P; II → Q; III → S; IV →T
(b) I → R; II → S; III → S; IV →T
(c) I → P; II → Q; III → Q; IV → R
(d) I → R; II → S; III → Q; IV → R
Ans. a
Q.4. Assuming 1μg of trace radioactive element X with a half life of 30 years is absorbed by a growing tree. The amount of X remaining in the tree after 100 years is ______ × 10−1μg.
[Given: ln 10 = 2.303; log 2 = 0.30] (JEE Main 2022)
Ans. 1
Q.5. The reaction between X and Y is first order with respect to X and zero order with respect to Y.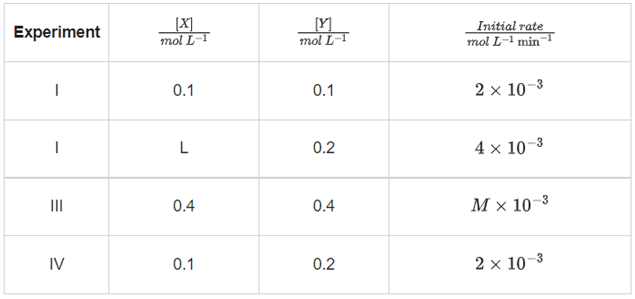 Examine the data of table and calculate ratio of numerical values of M and L. (Nearest Integer) (JEE Main 2022)
Examine the data of table and calculate ratio of numerical values of M and L. (Nearest Integer) (JEE Main 2022)
Ans. 40
r = k[X][Y]0 = k[X]
Using I & II
Using I & III
Q.6. For a reaction, given below is the graph of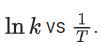 . The activation energy for the reaction is equal to ____________ calmol−1. (nearest integer)
. The activation energy for the reaction is equal to ____________ calmol−1. (nearest integer)
(Given: R = 2calK−1 mol−1) (JEE Main 2022)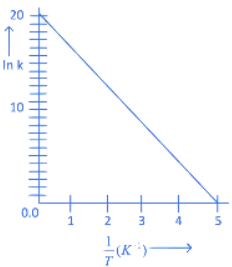
Ans. 8
Slope K = Ae−Ea/RT
Ea = 4R = 8Cal/mol
Q.7. For the given first order reaction
A → B
the half life of the reaction is 0.3010 min. The ratio of the initial concentration of reactant to the concentration of reactant at time 2.0 min will be equal to ___________. (Nearest integer) (JEE Main 2022)
Ans. 100
K = 2.303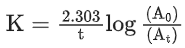
A0 → initial concentration of reactant
At → concentration of reactant at time t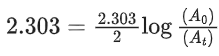
Q.8. Ka for butyric acid (C3H7COOH) is 2 × 10−5. The pH of 0.2M solution of butyric acid is __________ × 10−1. (Nearest integer)
[Given log2 = 0.30] (JEE Main 2022)
Ans. 27
Ka of Butyric acid ⇒ 2 × 10−5PKa = 4.7
pH of 0.2M solution
= 2.35 + 0.35 = 2.7
pH = 27 × 10−1
Q.9. 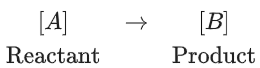
If formation of compound [B] follows the first order of kinetics and after 70 minutes the concentration of [A] was found to be half of its initial concentration. Then the rate constant of the reaction is x × 10−6 s−1. The value of x is ______________. (Nearest Integer) (JEE Main 2022)
Ans. 165
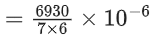
= 165 × 10−6 s−1
Q.10. 2NO + 2H2 → N2 + 2H2O
The above reaction has been studied at 800∘C. The related data are given in the table below: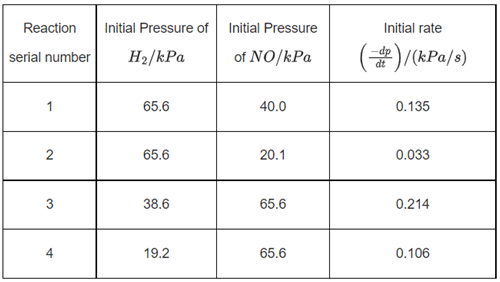 The order of the reaction with respect to NO is ___________. (JEE Main 2022)
The order of the reaction with respect to NO is ___________. (JEE Main 2022)
Ans. 2
Let the rate of reaction (r) is as
r = K[NO]n[H2]m
From 1st data
0.135 = K[40]n ⋅ (65.6)m...(1)
From 2nd data
0.033 = K(20.1)n ⋅ (65.6)m...(2)
On dividing equation (1) by equation (2)
4 = (2)n
∴ n = 2
∴ Order of reaction w.r.t. NO is 2.
Q.11. For a reaction A → 2 B + C the half lives are 100 s and 50 s when the concentration of reactant A is 0.5 and 1.0 mol L−1 respectively. The order of the reaction is ______________ . (Nearest Integer) (JEE Main 2022)
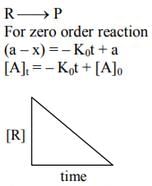
Ans. 2
t1/2 = 100sec a0 = 0.5
t1/2 = 50sec a0 = 1
(2) = (2)n−1
n − 1 = 1
n = 2
Q.12. For the decomposition of azomethane.
CH3N2CH3(g) → CH3CH3(g) + N2(g), a first order reaction, the variation in partial pressure with time at 600 K is given as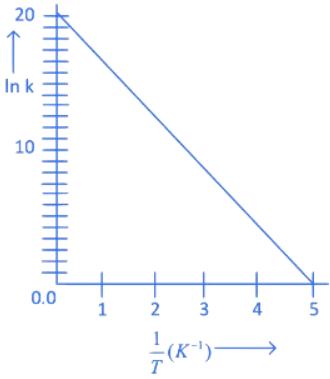 The half life of the reaction is __________ × 10−5 s. [Nearest integer] (JEE Main 2022)
The half life of the reaction is __________ × 10−5 s. [Nearest integer] (JEE Main 2022)
Ans. 2
For first order reaction,
ln A = ln A0 − kt
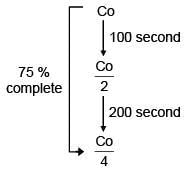
Hence Slope = −k
−k = −3.465 × 104
t1/2 = 2 × 10−5 s
Q.13. The half life for the decomposition of gaseous compound A is 240 s when the gaseous pressure was 500 Torr initially. When the pressure was 250 Torr, the half life was found to be 4.0 min. The order of the reaction is ______________. (Nearest integer) (JEE Main 2022)
Ans. 1
(t1/2)A = 240 s when P = 500 torr
(t1/2)A = 4min = 4 × 60 = 240 sec when P = 250 torr
If means half-life is independent of concentration of reactant present.
∴ Order of reaction = 1
Q.14. For the reaction P → B, the values of frequency factor A and activation energy EA are 4 × 1013 s−1 and 8.3 kJ mol−1 respectively. If the reaction is of first order, the temperature at which the rate constant is 2 × 10−6 s−1 is _____________ × 10−1 K.
(Given: ln 10 = 2.3, R = 8.3 J K−1 mol−1, log2 = 0.30) (JEE Main 2022)
Ans. 225
Q.15. The equation
k = (6.5 × 1012s−1)e−26000K/T
is followed for the decomposition of compound A. The activation energy for the reaction is ________ kJ mol−1. [nearest integer]
(Given: R = 8.314 J K−1 mol−1) (JEE Main 2022)
Ans. 216
Q.16. The activation energy of one of the reactions in a biochemical process is 532611 J mol−1. When the temperature falls from 310 K to 300 K, the change in rate constant observed is k300 = x × 10−3 k310. The value of x is ____________.
[Given: ln10 = 2.3, R = 8.3 J K−1 mol−1] (JEE Main 2022)
Ans. 1
Q.17. A radioactive element has a half life of 200 days. The percentage of original activity remaining after 83 days is ___________. (Nearest integer)
(Given: antilog 0.125 = 1.333, antilog 0.693 = 4.93) (JEE Main 2022)
Ans. 75
Q.18. For a first order reaction A → B, the rate constant, k = 5.5 × 10−14 s−1. The time required for 67% completion of reaction is x × 10−1 times the half life of reaction. The value of x is _____________ (Nearest integer)
(Given: log 3 = 0.4771) (JEE Main 2022)
Ans. 16
Q.19. The solubility product of a sparingly soluble salt A2X3 is 1.1 × 10−23. If specific conductance of the solution is 3 × 10−5 S m−1, the limiting molar conductivity of the solution is x× 10−3 S m2 mol−1. The value of x is ___________. (JEE Main 2022)
Ans. 3
Q.20. It has been found that for a chemical reaction with rise in temperature by 9 K the rate constant gets doubled. Assuming a reaction to be occurring at 300 K, the value of activation energy is found to be ____________ kJ mol−1. [nearest integer]
(Given ln10 = 2.3, R = 8.3 J K−1 mol−1, log 2 = 0.30) (JEE Main 2022)
Ans. 59
Q.21. pH value of 0.001 M NaOH solution is ____________. (JEE Main 2022)
Ans. 11
Q.22. The rate constant for a first order reaction is given by the following equation: 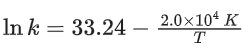
The activation energy for the reaction is given by ____________ kJ mol−1. (In nearest integer) (Given: R = 8.3 J K−1 mol−1) (JEE Main 2022)
Ans. 166
Q.23. Catalyst A reduces the activation energy for a reaction by 10 kJ mol−1 at 300 K. The ratio of rate constants,  is ex. The value of x is ___________. [nearest integer]
is ex. The value of x is ___________. [nearest integer]
[Assume that the pre-exponential factor is same in both the cases. Given R = 8.31 J K−1 mol−1] (JEE Main 2022)
Ans. 4
Q.24. A flask is filled with equal moles of A and B. The half lives of A and B are 100 s and 50 s respectively and are independent of the initial concentration. The time required for the concentration of A to be four times that of B is ___________ s.
(Given: ln 2 = 0.693) (JEE Main 2022)
Ans. 200
Q.25. At 345 K, the half life for the decomposition of a sample of a gaseous compound initially at 55.5 kPa was 340 s. When the pressure was 27.8 kPa, the half life was found to be 170 s. The order of the reaction is ____________. [integer answer] (JEE Main 2022)
Ans. 0
Q.26. For a given chemical reaction
γ1A + γ2B → γ3C + γ4D
Concentration of C changes from 10 mmol dm−3 to 20 mmol dm−3 in 10 seconds. Rate of appearance of D is 1.5 times the rate of disappearance of B which is twice the rate of disappearance A. The rate of appearance of D has been experimentally determined to be 9 mmol dm−3 s−1. Therefore, the rate of reaction is _____________ mmol dm−3 s−1. (Nearest Integer) (JEE Main 2022)
Ans. 1
r2 = 2r1
r4 = 1.5r2 = 3r1
r4 = 9r3 = 3r1
⇒ r1 = 3r3
3r3 A + 6r3 B → r3C + 9r3D
= 1 m⋅mol dm−3sec−1
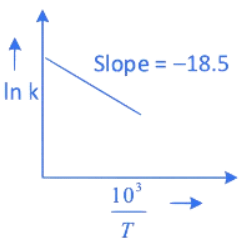
Q.27. The rate constants for decomposition of acetaldehyde have been measured over the temperature range 700 - 1000 K. The data has been analysed by plotting ln k vs 103 / T graph. The value of activation energy for the reaction is ___________ kJ mol−1. (Nearest integer)
(Given: R = 8.31 J K−1 mol−1) (JEE Main 2022)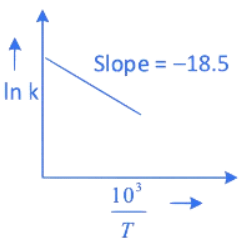
Ans. 154
∴ Ea = 18.5 × 8.31 × 1000 ≃ 154 kJ mol−1
Q.28. At 30∘C, the half life for the decomposition of AB2 is 200 s and is independent of the initial concentration of AB2. The time required for 80% of the AB2 to decompose is
Given: log 2 = 0.30 log 3 = 0.48 (JEE Main 2022)
(a) 200 s
(b) 323 s
(c) 467 s
(d) 532 s
Ans. c
Since, half-life is independent of the initial concentration of AB2. Hence, the reaction is "First Order".
t = 467 s
Q.29. Ka1, Ka2 and Ka3 are the respective ionization constants for the following reactions (a), (b) and (c).
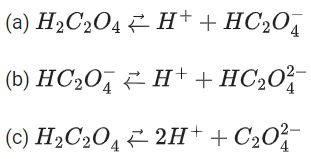
The relationship between Ka1, Ka2 and Ka3 is given as (JEE Main 2022)
(a) Ka3 = Ka1 + Ka2
(b) Ka3 = Ka1 − Ka2
(c) Ka3 = Ka1 / Ka2
(d) Ka3 = Ka1 × Ka2
Ans. d
Ka3 = Ka1 × Ka2
Q.30. The equilibrium constant for the reversible reaction
2A(g) ⇌ 2B(g) + C(g) is K1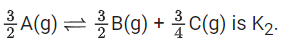
K1 and K2 are related as: (JEE Main 2022)
(a)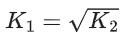
(b)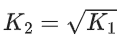
(c)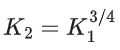
(d)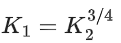
Ans. c
Q.31. The solubility of AgCl will be maximum in which of the following? (JEE Main 2022)
(a) 0.01 M KCl
(b) 0.01 M HCl
(c) 0.01 M AgNO3
(d) Deionised water
Ans. d
In deionized water no common ion effect will take place so maximum solubility.
Q.32. For a first order reaction, the time required for completion of 90% reaction is 'x' times the half life of the reaction. The value of 'x' is
(Given: ln 10 = 2.303 and log 2 = 0.3010) (JEE Main 2022)
(a) 1.12
(b) 2.43
(c) 3.32
(d) 33.31
Ans. c
A → Products
For a first order reaction,
Time for 90% conversion,
Q.33. For the following reaction,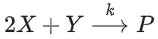 the rate of reaction is
the rate of reaction is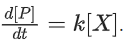 Two moles of X are mixed with one mole of Y to make 1.0 L of solution. At 50 s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are)
Two moles of X are mixed with one mole of Y to make 1.0 L of solution. At 50 s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are)
(Use: ln 2 = 0.693) (JEE Advanced 2021)
(a) The rate constant, k, of the reaction is 13.86 × 10−4 s−1.
(b) Half-life of X is 50 s.
(c)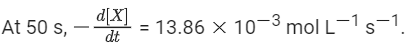
(d)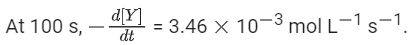
Ans. b, c, d
As the concentration of reactant becomes half at t = 50 s. So, half-time of reaction is 50 s.
Given,
So, options (b), (c) and (d) are correct.
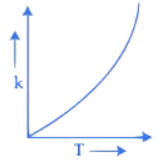
Q.34. Which one of the following given graphs represents the variation of rate constant (k) with temperature (T) for an endothermic reaction? (JEE Main 2021)
(a)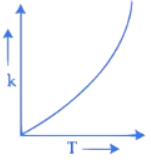
(b)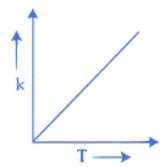
(c)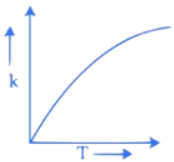
(d)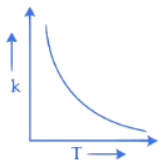
Ans. a
According to Arrhenius equation
The graph will varies as
Q.35. For a reaction of order n, the unit of the rate constant is: (JEE Main 2021)
(a) A mol1−n L1−n s
(b) mol1−n L2n s−1
(c) mol1−n Ln−1 s−1
(d) mol1−n L1−n s−1
Ans. c
Rate = k[A]n
comparing units
⇒ k = mol1−n L1−n s−1
Q.36. For the following graphs, 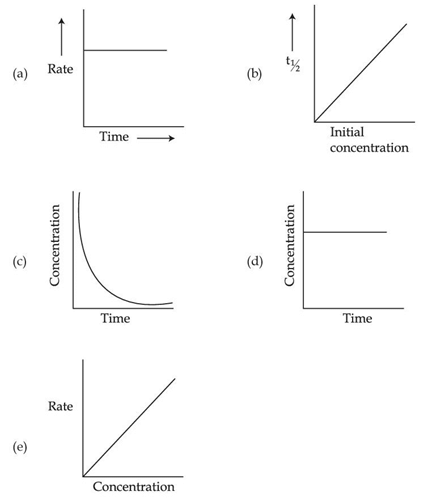 Choose from the options given below, the correct one regarding order of reaction is: (JEE Main 2021)
Choose from the options given below, the correct one regarding order of reaction is: (JEE Main 2021)
(a) (b) Zero order (c) and (e) First order
(b) (a) and (b) Zero order (e) First order
(c) (b) and (d) Zero order (e) First order
(d) (a) and (b) Zero order (c) and (e) First order
Ans. b
For Zero order reactions
rate = K [Reactant]0
⇒ r = k
For First order reaction →
r = K [Concentration]
Reactant concentration after time t →
Q.37. Isotope(s) of hydrogen which emits low energy β− particles with t1/2 value > 12 years is/are (JEE Main 2021)
(a) Protium
(b) Tritium
(c) Deuterium
(d) Deuterium and Tritium
Ans. b
Q.38. Given below are two statements:
Statement I: The pH of rainwater is normally − 5.6.
Statement II: If the pH of rainwater drops below 5.6, it is called acid rain.
In the light of the above statements, choose the correct answer from the options given below: (JEE Main 2021)
(a) Both Statement I and Statement II are true.
(b) Both Statement I and Statement II are false.
(c) Statement I is true but Statement II is false.
(d) Statement I is false but Statement II is true.
Ans. a
The pH of rain water is normally 5.6. If it pH drop below 5.6, it is called acid rain.
∴ Both statements are correct.
Q.39. For the reaction A → B, the rate constant k(in s−1) is given by 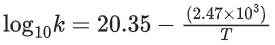
The energy of activation in kJ mol−1 is ____________. (Nearest integer) [Given: R = 8.314 J K−1 mol−1] (JEE Main 2021)
Ans. 47
Given,
We know
= 47.29 = 47 (Nearest integer)
Q.40. According to the following figure, the magnitude of the enthalpy change of the reaction
A + B → M + N in kJ mol− is equal to ___________. (Integer answer) (JEE Main 2021) 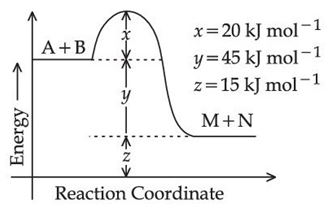
Ans. 45
ΔH = EProduct − EReactant
= 15 − (15 + 45)
= −45 KJ/mol
|ΔH| = 45 KJ/mol
Q.41. For a first order reaction, the ratio of the time for 75% completion of a reaction to the time for 50% completion is ____________. (Integer answer) (JEE Main 2021)
Ans. 2
Q.42. The first order rate constant for the decomposition of CaCO3 at 700 K is 6.36 × 10−3s−1 and activation energy is 209 kJ mol−1. Its rate constant (in s−1) at 600 K is x × 10−6. The value of x is ___________. (Nearest integer)
[Given R = 8.31 J K− mol−1; log 6.36 × 10−3 = −2.19, 10−4.79 = 1.62 × 10−5] (JEE Main 2021)
Ans. 16
K700 = 6.36 × 10−3s−1;
K600 = x × 10−6s−1
Ea = 209 kJ/mol
Applying;
log(6.36 × 10−3) − logK600 = 2.6
⇒ logK600 = −2.19 − 2.6 = −4.79
⇒ K600 = 10−4.79 = 1.62 × 10−5
= 1.62 × 10−6
= x × 10−6
⇒ x = 16
Q.43. The reaction that occurs in a breath analyser, a device used to determine the alcohol level in a person's blood stream is
2K2Cr2O7 + 8H2SO4 + 3C2H6O → 2Cr2(SO4)3 + 3C2H4O2 + 2K2SO4 + 11H2O
If the rate of appearance of Cr2(SO4)3 is 2.67 mol min−1 at a particular time, the rate of disappearance of C2H6O at the same time is _____________ mol min−1. (Nearest integer) (JEE Main 2021)
Ans. 4
⇒ Rate of disappearance of C2H6O = 4.005 mol/min.
Q.44. The reaction rate for the reaction
[PtCl4]2− + H2O ⇌ [Pt(H2O)Cl3]− + Cl−
was measured as a function of concentrations of different species. It was observed that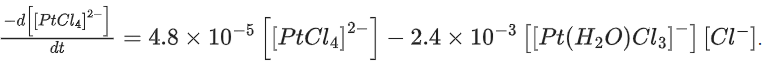
where square brackets are used to denote molar concentrations. The equilibrium constant Kc = ____________ . (Nearest integer) (JEE Main 2021)
Ans. 50
[PtCl4]2− + H2O ⇌ [Pt(H2O)Cl3]− + Cl−
Q.45. The overall stability constant of the complex ion [Cu(NH3)4]2+ is 2.1 × 1013. The overall dissociations constant is y × 10−14. Then y is __________. (Nearest integer) (JEE Main 2021)
Ans. 5
Given ks = 2.1 × 1013
Kd = 1/ks = 4.7 × 10−14
∴ y = 4.7 ≈ 5
Q.46. The following data was obtained for chemical reaction given below at 975 K.
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)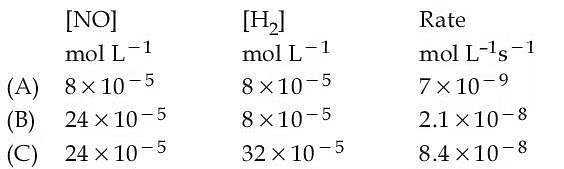 The order of the reaction with respect to NO is ___________. [Integer answer] (JEE Main 2021)
The order of the reaction with respect to NO is ___________. [Integer answer] (JEE Main 2021)
Ans. 1
7 × 10−9 = K × (8 × 10−5)x (8 × 10−5)y ......... (1)
2.1 × 10−8 = K × (24 × 10−5)x (8 × 10−5)y ....... (2)
Q.47. For the first order reaction A → 2B, 1 mole of reactant A gives 0.2 moles of B after 100 minutes. The half life of the reaction is __________ min. (Round off to the nearest integer).
[Use: ln 2 = 0.69, ln 10 = 2.3]
Properties of logarithms: ln xy = y ln x;
ln(x/y) = nx − lny
(Round off to the nearest integer) (JEE Main 2021)
Ans. Between 600 and 700
Ans. 600 to 700
Q.48. For a chemical reaction A → B, it was found that concentration of B is increased by 0.2 mol L− in 30 min. The average rate of the reaction is ____________ × 10−1 mol L−1 h−1. (in nearest integer) (JEE Main 2021)
Ans. 4
A → B
= 0.4 = 4 × 10−1 mol / L × hr
Q.49. 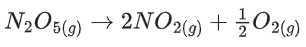
In the above first order reaction the initial concentration of N2O5 is 2.40 × 10−2 mol L−1 at 318 K. The concentration of N2O5 after 1 hour was 1.60 × 10−2 mol L−1. The rate constant of the reaction at 318 K is ______________ × 10−3 min−1. (Nearest integer)
[Given: log 3 = 0.477, log 5 = 0.699] (JEE Main 2021)
Ans. 7
Q.50. PCl5(g) → PCl3(g) + Cl2(g)
In the above first order reaction the concentration of PCl5 reduces from initial concentration 50 mol L−1 to 10 mol L−1 in 120 minutes at 300 K. The rate constant for the reaction at 300 K is x × 10−2 min−1. The value of x is __________. [Given log5 = 0.6989] (JEE Main 2021)
Ans. 1
t = 0
50M
t = 120min
10 M
= 1.3413 × 10−2 min−1
1.34 ⇒ Nearest integer = 1
Q.51. The inactivation rate of a viral preparation is proportional to the amount of virus. In the first minute after preparation, 10% of the virus is inactivated. The rate constant for viral inactivation is ___________ × 10−3 min−1. (Nearest integer)
[Use: ln 10 = 2.303; log10 3 = 0.477; property of logarithm : log xy = y log x] (JEE Main 2021)
Ans. 106
Unit of rate constant is min−1, so it must be a first order reaction. For first order reaction,
k is the rate constant
t is the time
A0 is the initial conc.
At is the conc. at time, t
Using formula,
A0 = 100, At = 90 min 1 min
= 2.303 × (log 10 − 2 log 3)
= 2.303 × (1 − 2 × 0.477)
= 0.10593
= 105.93 × 10−3
≈ 106
Hence, the rate constant for viral inactivation is 106.
Q.52. A reaction has a half life of 1 min. The time required for 99.9% completion of the reaction is _________ min. (Round off to the Nearest Integer). [Use: ln 2 = 0.69; ln 10 = 2.3] (JEE Main 2021)
Ans. 10
Given t1/2 = 1 min
From formula we know,
⇒ t = 10 min
Q.53. 2NO(g) + Cl2(g) ⇌ 2NOCl(s)
This reaction was studied at −10∘ and the following data was obtained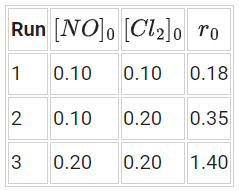 [NO]0 and [Cl2]0 are the initial concentrations and r0 is the initial reaction rate.
[NO]0 and [Cl2]0 are the initial concentrations and r0 is the initial reaction rate.
The overall order of the reaction is __________. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 3
We know,
Rate = K[A]x[B]y
Here from,
Exp 1 : 0.18 = K[0.1]x[0.1]y ..... (1)
Exp. 2 : 0.35 = K[0.1]x[0.2]y .... (2)
Exp. 3 : 1.40 = K[0.2]x[0.2]y .... (3)
(2) ÷ (3)
⇒ x = 2
(1) ÷ (2)
⇒ y = 1
∴ x + y = 3
Q.54. The reaction 2A + B2 → 2AB is an elementary reaction.
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of 3, the rate of the reaction increases by a factor of ____________. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 27
Let initial concentration of A & B is a & b
Q.55. For a certain first order reaction 32% of the reactant is left after 570s. The rate constant of this reaction is _________ × 10−3 s−1. (Round off to the Nearest Integer). [Given: log102 = 0.301, ln10 = 2.303] (JEE Main 2021)
Ans. 2
k = 2 × 10−3s−1
Q.56. A and B decompose via first order kinetics with half-lives 54.0 min and 18.0 min respectively. Starting from an equimolar non reactive mixture of A and B, the time taken for the concentration of A to become 16 times that of B is _________ min. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 108
Initially : [A0] = [B0] = a
After time 't' min : [A] = 16[B]
Q.57. The decomposition of formic acid on gold surface follows first order kinetics. If the rate constant at 300 K is 1.0 × 10−3 s−1 and the activation energy Ea = 11.488 kJ mol−1, the rate constant at 200 K is ____________ × 10−5 s−1. (Round off to the Nearest Integer). (Given : R = 8.314 J mol−1 K−1) (JEE Main 2021)
Ans. 10
k1 (at 200 K) = ?
k2 (at 300 K) = 1 × 10−3s−1
⇒ k1 = 10 × 10−5s−1
Q.58. If the activation energy of a reaction is 80.9 kJ mol−1, the fraction of molecules at 700 K, having enough energy to react to form products is e−x. The value of x is __________. (Rounded off to the nearest integer) [Use R = 8.31 J K−1 mol−1] (JEE Main 2021)
Ans. 14
Ea = 80.9 kJ/mol
Fraction of molecules able to cross energy barrier = e−Ea/RT = e−x
⇒ x ≃ 14
Q.59. The rate constant of a reaction increases by five times on increase in temperature from 27∘C to 52∘C. The value of activation energy in kJ mol−1 is _________. (Rounded off to the nearest integer)
[R = 8.314 J K−1mol−1] (JEE Main 2021)
Ans. 52
T1 = (273 + 27) = 300 K, T2 = (273 + 52) = 325 K
Given, temperature coefficient of the reaction,
Ea = 52194.78 J mol−
= 52.194 kJ mol−1
≃ 52 kJ mol−1
Q.60. For the reaction, aA + bB → cC + dD, the plot of log k vs 1/T is given below: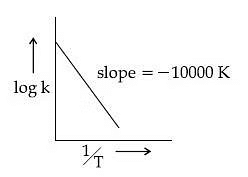
The temperature at which the rate constant of the reaction is 10-4 s-1 is _________ K. (Rounded off to the nearest integer)
[Given: The rate constant of the reaction is 10-5 s-1 at 500 K.] (JEE Main 2021)
Ans. 526
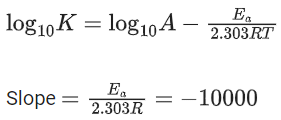
Q.61. Sucrose hydrolyses in acid solution into glucose and fructose following first order rate law with a half-life of 3.33 h at 25∘C. After 9 h, the fraction of sucrose remaining is f. The value of log10(1/f) is ________ × 10−2. (Rounded off to the nearest integer)
[Assume: ln 10 = 2.303, ln 2 = 0.693] (JEE Main 2021)
Ans. 81
At t = 0, a = [A]0 (initial conc.)
t = 9h, a − x = [A]t [conc. at time t]
For using 1st order equation,
x = 81.24 or x ≈ 81
Q.62. Gaseous cyclobutene isomerises to butadiene in a first order process which has a 'k' value of 3.3 × 10-4 s-1 at 153°C. The time in minutes it takes for the isomerization to proceed 40% to completion at this temperature is ______.
(Rounded off to the nearest integer) (JEE Main 2021)
Ans. 26
It is a first order isomerisation reaction. Integrated rate law for 1st order reaction is
Here,
k = rate constant
[A]0 = initial concentration
[A]t = concentration at time 't'
Given, k = 3.3 × 10−4 s−1
[A]0 = 100 ⇒ [A]t = 100 − 40 = 60
Put values in Eq. (i), we get
t = 1547.95 s = 25.79 min (1 min = 60 s or 1s = 1/60 min) = 26 minutes
Q.63. During the nuclear explosion, one of the products is 90Sr with half-life of 6.93 years. If 1 µg of 90Sr was absorbed in the bones of a newly born baby in place of Ca, how much time, in years, is required to reduce it by 90% if it is not lost metabolically? (2020)
Ans. 23.03
Given, half-life of 90Sr = 6.93 year; amount = 1 × 10–6g
From the first order kinetic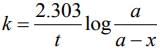
For 90% decay of 90Sr,
⇒ t = 23.03 log 10
⇒ t = 23.03 years
Q.64. For the reaction
2H2(g) + 2NO(g) → N2(g) + 2H2O(g),
the observed rate expression is, rate =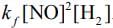 . The rate expression for the reverse reaction is (2020)
. The rate expression for the reverse reaction is (2020)
(a) kb[N2][H2O]2
(b) 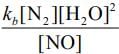
(c) kb [N2][H2O]
(d) 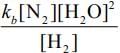
Ans. d
Given, rate expression for reaction,
2H2 (g) + 2NO(g) → N2(g) + 2H2O(g)rf=kf[NO]2[H2]
Now,
⇒Rearranging the above equation,
=> rf = rb
Q.65. The rate of a certain biochemical reaction at physiological temperature (T) occurs 106 times faster with enzyme than without it. The change in the activation energy upon adding enzyme is (2020)
(a) −6(2.303)RT
(b) −6RT
(c) +6(2.303)RT
(d) +6RT
Ans. a
From Arrhenius equation without enzyme
(1)
With enzyme(2)
On Dividing Eq. (1) by (2), we get
⇒(2)
Taking log of Eq. (3), we get
E'a - Ea = - 6(2.303)RT
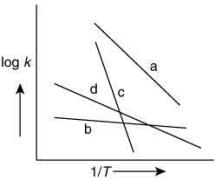
Q.66.Consider the following plots of rate constant versus 1/T for four different reactions. Which of the following orders is correct for the activation energies of these reactions? (2020)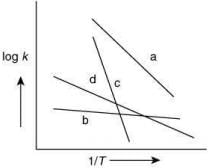
(a) Eb > Ea > Ed > Ec
(b) Ea > Ec > Ed > Eb
(c) Ec > Ea > Ed > Eb
(d) Eb > Ed > Ec > Ea
Ans. c
From Arrhenius equation without enzyme
Taking log of Eq. (1), we get
By plotting log k against 1/T, the slope =and intercept = log A
From the graph of four different reaction,
The plot having high value of slop will have high value of activation energy. Thus, the correct order for activation energy of the reactions is: Ec > Ea > Ed > Eb.
Q.67. For the following reactions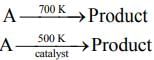
it was found that the Ea is decreased by 30 kJ mol–1 in the presence of catalyst. If the rate remains unchanged, the activation energy for catalyzed reaction is (Assume pre exponential factor is same) (2020)
(a) 75 kJ mol–1
(b) 105 kJ mol–1
(c) 135 kJ mol–1
(d) 198 kJ mol–1
Ans. a
Given, Eal = Ea and Ea2 = Ea - 30
T1 = 700 K and T2 = 500 K(1) [Given, Rate remains unchanged]
Substituting the values in Eq. (1), we get
⇒ 500Ea = 700Ea - 2100
⇒ 2100 = 700Ea - 500Ea
⇒ 2100 = 200Ea
⇒
⇒ Ea = 105 KJ mol-1
Thus, activation energy in presence of catalyst is Ea2 = Ea - 30
⇒ Ea2 = 105 - 30 = 75 kJ mol-1
Q.68. A sample of milk splits after 60 min. at 300 K and after 40 min. at 400 K when the population of lactobacillus acidophilus in it doubles. The activation energy (in kJ mol−1) for this process is closest to ________. (2020)
Ans. 3.98
Using Arrhenius equation at two different temperature,
(1)
Since, milk splits after 60 min. at 300 K and after 40 min. at 400 K.
Substituting the values in Eq. (1), we get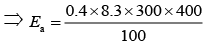
⇒ Ea = 3.984 kJ mol−1
Q.69. The following results were obtained during kinetic studies of the reaction;
2A + B → Products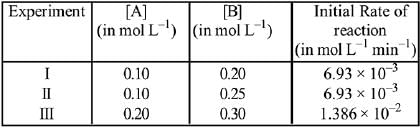
The time (in minutes) required to consume half of A is: (2019)
(a) 5
(b) 10
(c) 1
(d) 100
Ans. a
Q.70. For the reaction, 2A + B → products, when the concentrations of A and B both were doubled, the rate of the reaction increased from 0.3 mol L-1s-1 to 2.4 mol L-1s-1. When the concentration of A alone is doubled, the rate increased from 0.3 L-1s-1 to 0.6 mol L-1s-1. Which one of the following statements is correct? (2019)
(a) Total order of the reaction is 4
(b) Order of the reaction with respect to B is 2
(c) Order of the reaction with respect to B is 1
(d) Order of the reaction with respect to A is 2
Ans. b
∴ y = 2
Q.71. Consider the given plots for a reaction obeying Arrhenius equation (0°C < T < 300°C): (K and Ea are rate constant and activation energy, respectively)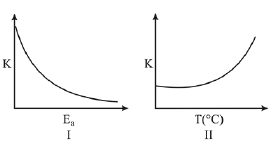
Choose the correct option: (2019)
(a) I is right but II is wrong
(b) Both I and II are correct
(c) I is wrong but II is right
(d) Both I and II are wrong
Ans. b
From Arrhenius equation,
K= Ae-Ea/RT
So, as Ea increases, e-Ea/RT decreases, K decreases and as T increases, Ea/RT decreases, e-Ea/RT increases,
Q.72. For an elementary chemical reaction, 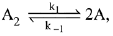 the expression for d[A]/dt is: (2019)
the expression for d[A]/dt is: (2019)
(a) k1[A2] - k-1[A]2
(b) 2k1[A2] - k-1[A]2
(c) k1 [A2] + k-1[A]2
(d) 2k1[A2] - 2k-1[A]2
Ans. d
⇒
Q.73. If a reaction follows the Arrhenius equation, the plot lnk vs 1/(RT) gives straight line with a gradient (-y) unit. The energy required to activate the reactant is: (2019)
(a) y/R unit
(b) y unit
(c) yR unit
(d) - y unit
Ans. b
From Arrhenius equation,
slope = -y (given)
-y = -Ea
⇒ Ea = y
Q.74. The reaction 2X → B is a zeroth order reaction. If the initial concentration of X is 0.2 M, the half-life is 6 h. When the initial concentration of X is 0.5 M, the time required to reach its final concentration of 0.2 M will be: (2019)
(a) 9.0 h
(b) 12.0 h
(c) 18.0 h
(d) 7.2 h
Ans. c
For zero order reaction
t = 18 hrs
Q.75. Decomposition of X exhibits a rate constant of 0.05 μg/year. How many years are required for the decomposition of 5 μg of X into 2.5 μg? (2019)
(a) 50
(b) 25
(c) 20
(d) 40
Ans. a
Rate constant of decomposition of X= 0.05 mg/year. Unit of rate constant confirms that the decomposition of X is a zero order reaction.
For zero order kinetics,
Q.76. For a reaction, consider the plot of In k versus 1/T given in the figure. If the rate constant of this reaction at 400 K is 10-5 s-1, then the rate constant at 500 K is: (2019)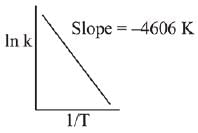 (a) 10-6 s-1
(a) 10-6 s-1
(b) 2 x 10-4 s-1
(c) 10-4 s-1
(d) 4 x 10-4 s-2
Ans. c
From Arrhenius equation,
Q.77. For the reaction 2A + B → C, the values of initial rate at different reactant concentrations are given in the table below.
The rate law for the reaction is: (2019)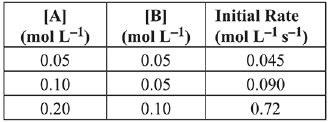 (a) Rate = k[A][B]2
(a) Rate = k[A][B]2
(b) Rate = k[A]2[B]2
(c) Rate = k[A][B]
(d) Rate = k[A]2[B]
Ans. a
2A + B → C
Rate = k[A]x [B]y
Exp-1, 0.045 = k[0.05]x [0.05]y ...(i)
Exp-2, 0.090 = k[0.1]x [0.05]y ...(ii)
Exp-3, 0.72 = k[0.2]x [0.1]y ...(iii)
Divide equation (i) by equation (ii)
Divide equation (i) by equation (iii)
Rate law = k[A]1 [B]2.
Q.78. For a reaction scheme 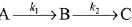 if the rate of formation of B is set to be zero then the concentration of B is given by: (2019)
if the rate of formation of B is set to be zero then the concentration of B is given by: (2019)
(a) (k1 - k2) [A]
(b) k1k2 [A]
(c) (k1 + k2) [A]
(d) (k1/k2) [A]
Ans. d
Q.79. The given plots represents the variation of the concentration of a reactant R with time for two different reactions (i) and (ii). The respective orders of the reactions are: (2019)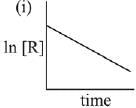
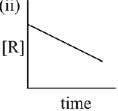 (a) 1, 0
(a) 1, 0
(b) 1, 1
(c) 0, 1
(d) 0, 2
Ans. a
For First order reaction
Q.80. A bacterial infection in an internal wound grows as N2(t) = N0 exp(t), where the time t is in hours. A dose of antibiotic, taken orally, needs 1 hour to reach the wound. Once it reaches there, the bacterial population goes down as dN/dt = -5N2. What will be the plot of N0/N vs. t after 1 hour? (2019)
(a) 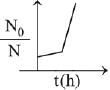
(b) 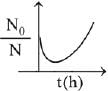
(c) 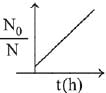
(d) 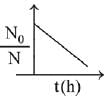
Ans. c
When drug is administered bacterial growth is given by dN/dt = -5N2
On integrating the above equation,
The above equation is similar to straight line equation with positive slope.
Thus N0/Nt increases linearly with t.
Q.81. For the reaction of H2 with I2, the rate constant is 2.5 x 10-4 dm3 mol-1 s-1 at 327°C and 1.0 dm3 mol-1 s-1 at 527°C. The activation energy for the reaction, in kJ mol-1 is: (R = 8.314J K-1 mol-1) (2019)
(a) 166
(b) 150
(c) 72
(d) 59
Ans. a
Q.82. In the following reaction: xA → yB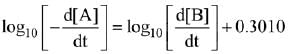
‘A' and ‘B’ respectively can be: (2019)
(a) n-Butane and Iso-butane
(b) C2H2 and C6H6
(c) C2H4 and C4H8
(d) N2O4 and NO2
Ans. c
xA → yB
Comparing this equation with the equation given in question. We get,
∴ x/y = 2
∴ The reaction is of type 2A → B.
Hence, option (c) is correct.
Q.83. NO, required for a reaction is produced by the decomposition of N2O5 in CCl4 as per the equation,
2N2O5(g) → 4NO2(g) + O2(g).
The initial concentration of N2O5 is 3.00 mol L-1 and it is 2.75 mol L-1 after 30 minutes. The rate of formation of NO2 is: (2019)
(a) 4.167 x 10-3 mol L-1 min-1
(b) 1.667 x 10-2 mol L-1 min-1
(c) 8.333 x 10-3 mol L-1 min-1
(d) 2.083 x 10-3 mol L-1 min-1
Ans. b
According to the question
Q.84. At 5180C, the rate of decomposition of a sample of gaseous acetaldehyde, initially at a pressure of 363 Torr, was 1.00 Torr s-1 when 5% had reacted and 0.5 Torr s-1 when 33% had reacted. The order of the reaction is: (2018)
(a) 2
(b) 3
(c) 1
(d) 0
Ans. a
Q.85. N2O5 decomposes to NO2 and O2 and follows first order kinetics. After 50 minutes, the pressure inside the vessel increases from 50 mm Hg to 87.5 mm Hg. The pressure of the gaseous mixture after 100 minute at constant temperature will be: (2018)
(a) 106.25 mm Hg
(b) 116.25 mm Hg
(c) 136.25 mm Hg
(d) 175.0 mm Hg
Ans. a
2N2O5 → 4NO2 + O2
p − 2x 4x x
pt = p − 2x + 4x + x
pt = p + 3x
at t = 0, pt = p = 50 mm Hg
at t = 50 mm, pt = 87.5 mm Hg
p + 3x = 87.5
p = 87.5 − 3x
50 = 87.5 − 3x
12.5 = x
p − 2x = 50 − 2(12.5) = 25
Since K will remain same
50 = 50 × 4 − 8y
50 = 200 − 8y
8y = 150
y = 18.75
pt = p + 3y
= 50 + 3 (18.73) = 106.25 mm Hg
Q.86. If 50 % of a reaction occurs in 100 second and 75 % of the reaction occurs in 200 second, the order of this reaction is: (2018)
(a) 2
(b) 3
(c) Zero
(d) 1
Ans. d
First order reaction as half life is constant.
Q.87. Two reactions R1 and R2 have identical pre - exponential factors. Activation energy of R1 exceeds that of R2 by 10 kJ mol–1. If k1 and k2 are rate constants for reactions R1 and R2 respectively at 300 K, then ln (k1/ k2) is equal to
(R = 8.314 J mole–1 K–1) (2017)
(a) 8
(b) 12
(c) 6
(d) 4
Ans. d
Q.88. The rate of a reaction A doubles on increasing the temperature from 300 to 310 K. By how much, the temperature of reaction B should be increased from 300 K so that rate doubles if activation energy of the reaction B is twice to that of reaction A. (2017)
(a) 4.92 K
(b) 9.84 K
(c) 19.67 K
(d) 2.45 K
Ans. a
...(i)
...(ii)
= 304.92
Q.89. The rate of a reaction quadruples when the temperature changes from 300 to 310 K. The activation energy of this reaction is:
(Assume activation energy and pre-exponential factor are independent of temperature; ln 2 = 0.693; R = 8.314 J mol-1 K-1) (2017)
(a) 53.6 kJ mol-1
(b) 214.4 kJ mol-1
(c) 107.2 kJ mol-1
(d) 26.8 kJ mol-1
Ans. c
= 107165.79 J = 107.165 KJ
Q.90. Decomposition of H2O2 follows a first order reaction. In fifty minutes the concentration of H2O2 decreases from 0.5 to 0.125 M in one such decomposition. When the concentration of H2O2 reaches 0.05 M, the rate of formation of O2 will be: (2016)
(a) 6.93 x 10-2 mol min-1
(b) 6.93 x 10-4 mol min-1
(c) 2.66 L min-1 at STP
(d) 1.34 x 10-2 mol min-1
Ans. b
For a first order reaction
K = 2.303/t log a/(a-x)
Given a = 0.5, (a-x) = 0.125, t = 50 min
∴ k = 2.303/50 log 0.5/0.125
= 2.78 x 10-2 min-1
r = d[H2O2] = 2.78 x 10-2 x 0.05
= 1.386 x 10-3 mol min-1
Now
Q.91. The reaction of ozone with oxygen atoms in the presence of chlorine atoms can occur by a two step process show below:
O3(g) + Cl*(g) → O2(g) + ClO*(g) ...(i)
ki = 5.2 × 109 L mol-1 s-1
ClO*(g) + O*(g) → O2(g) + Cl*(g) ...(ii)
[Kii = 2.6 x 1010 L mol-1s-1]
The closest rate constant for the overall reaction O3(g) + O*(g) → 2O2(g) is: (2016)
(a) 1.4 × 1020 L mol-1 s-1
(b) 5.2 × 109 L mol-1 s-1
(c) 3.1 × 1010 L mol-1 s-1
(d) 2.6 × 1010 L mol-1 s-1
Ans. b
The rate constant of overall reaction depends slowest step. Hence equation(i) is slowest step. Option(b) is correct.
Q.92. The rate law for the reaction below is given by the expression k [A][B]
A + B → Product
If the concentration of B is increased from 0.1 to 0.3 mole, keeping the value of A at 0.1 mole, the rate constant will be: (2016)
(a) 9 k
(b) 3 k
(c) k/3
(d) k
Ans. d
Rate constant is independent of concentration.