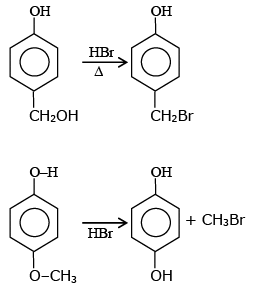
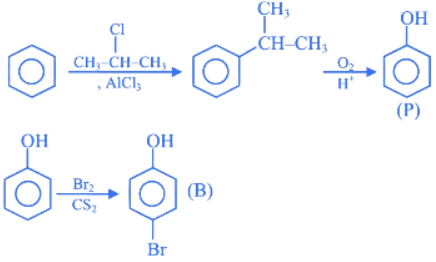
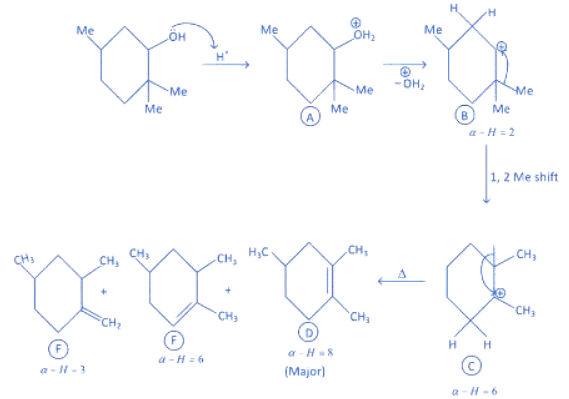
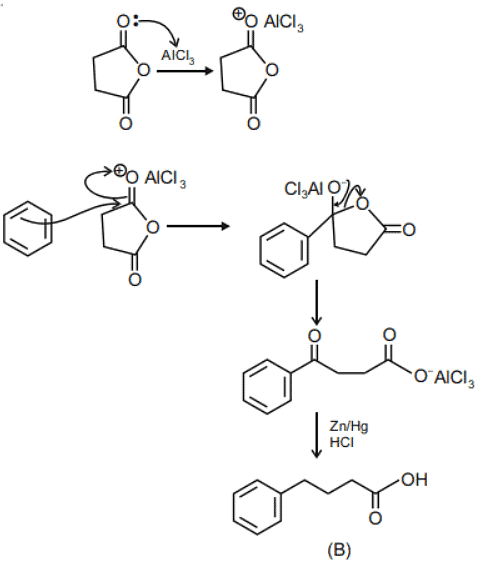
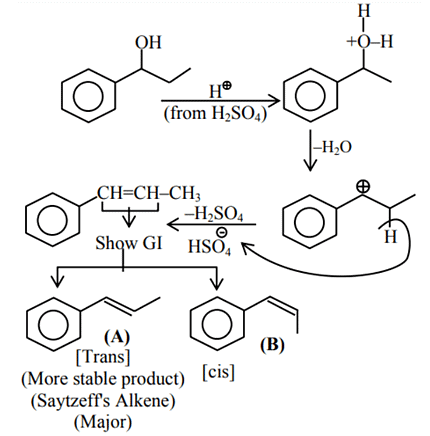
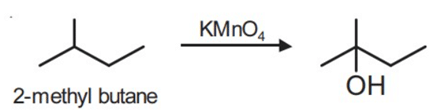
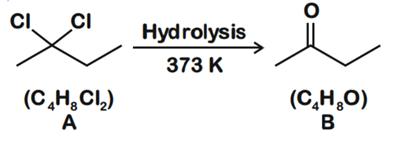
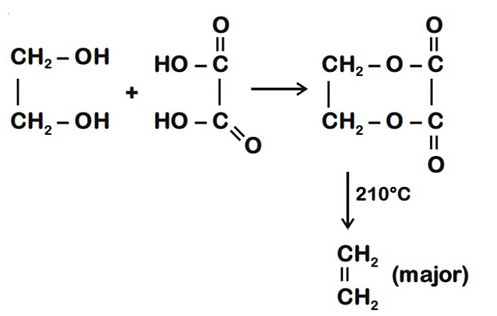
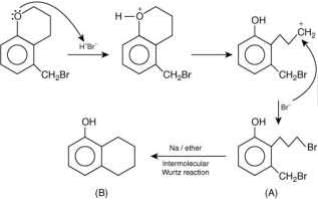
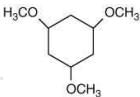
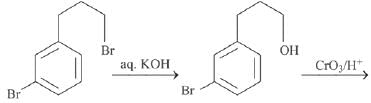
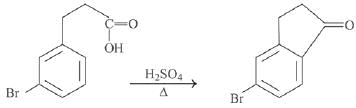
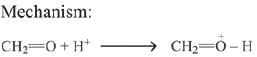
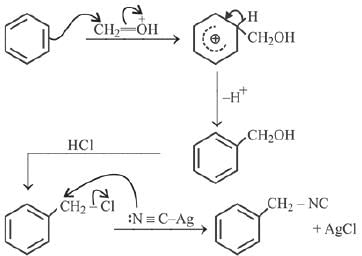
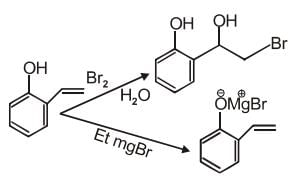
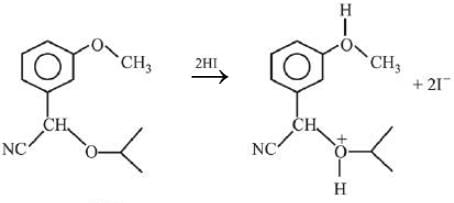
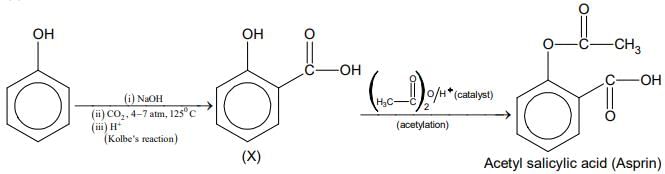
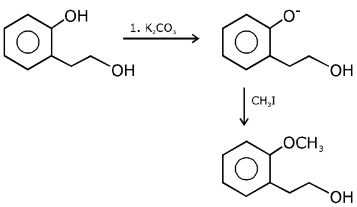
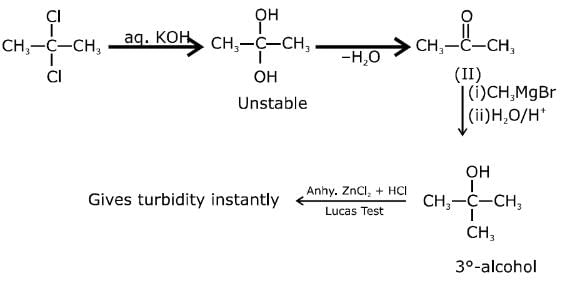
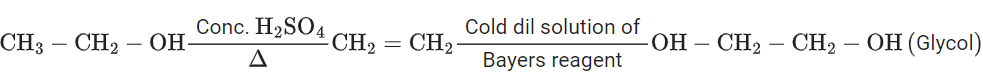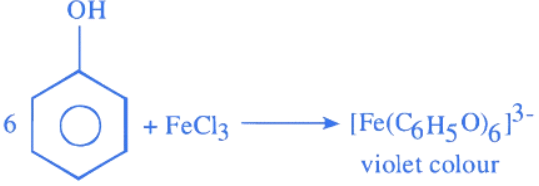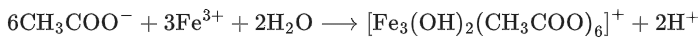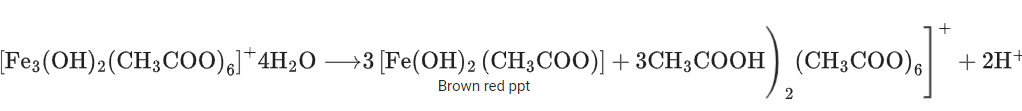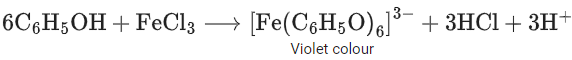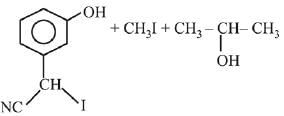Q.1. ' A ' and ' B ' formed in the following set of reactions are: (JEE Main 2023)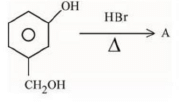
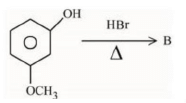
(a) 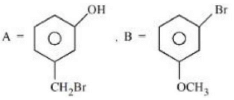
(b) 
(c) 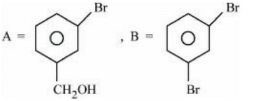
(d) 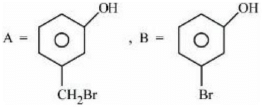
Ans. b
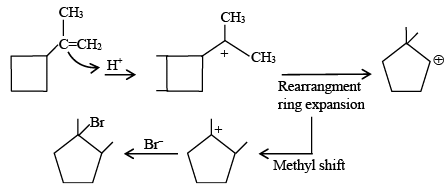
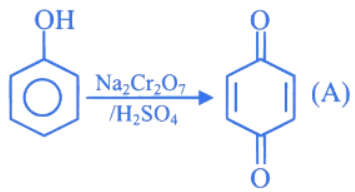
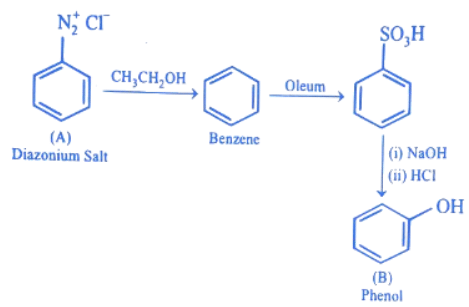
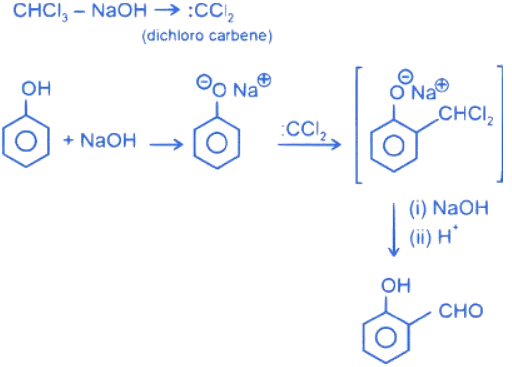
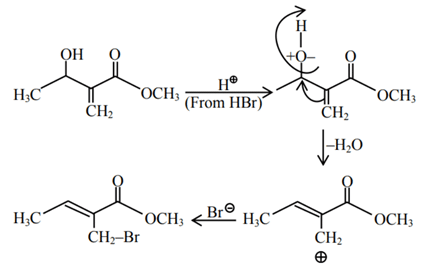
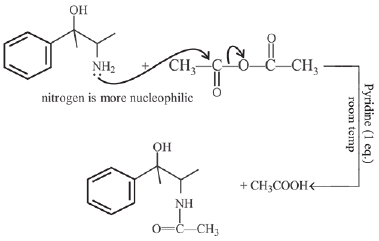
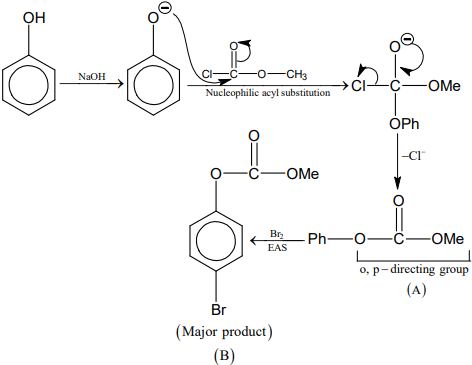
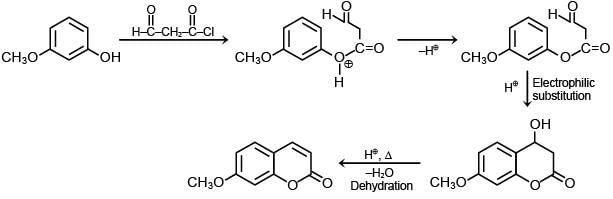
Q.2. In the following given reaction, ' A ' is (JEE Main 2023)
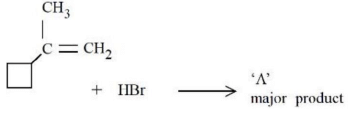 (a)
(a) 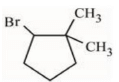
(b) 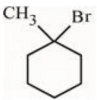
(c) 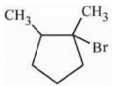
(d) 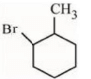
Ans. c
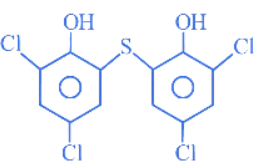
Q.3. The number of chlorine atoms in bithionol is __________. (JEE Main 2022)
Ans. 4
Number of chlorine atoms in bithionol = 4
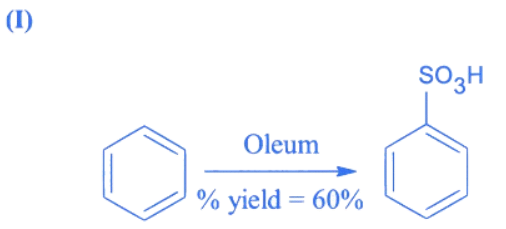
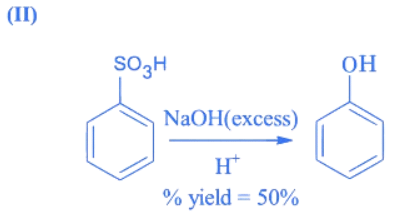
Q.4. In the following reaction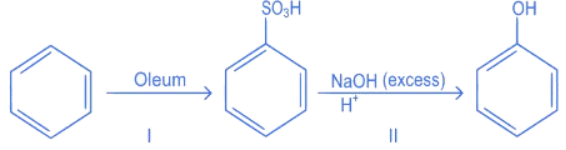 The % yield for reaction I is 60% and that of reaction II is 50%. The overall yield of the complete reaction is __________ %. [nearest integer] (JEE Main 2022)
The % yield for reaction I is 60% and that of reaction II is 50%. The overall yield of the complete reaction is __________ %. [nearest integer] (JEE Main 2022)
Ans. 30
Let initial moles of reactant taken = n
Total moles obtained for benzene sulphonic acid (with % yield = 60%) = 0.6n
Moles of benzene sulphonic acid before reaction II = 0.6n
Moles obtained for phenol (with % yield = 50%) = 0.6 × 0.5n = 0.3n
So over all % yield of complete reaction
Q.5. In the presence of sunlight, benzene reacts with Cl2 to give product, X. The number of hydrogens in X is _____________. (JEE Main 2022)
Ans. 6
Q.6. In the given reaction, 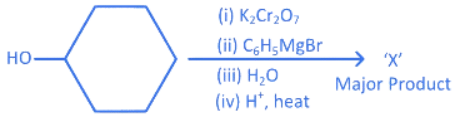 the number of sp2 hybridised carbon(s) in compound 'X' is ________. (JEE Main 2022)
the number of sp2 hybridised carbon(s) in compound 'X' is ________. (JEE Main 2022)
Ans. 8
Q.7. The number of chiral alcohol(s) with molecular formula C4H10O is ________. (JEE Main 2022)
Ans. 2
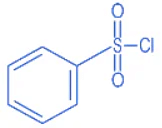
Q.8. The Hinsberg reagent is (JEE Main 2022)
(a)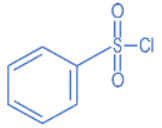
(b)
(c)
(d)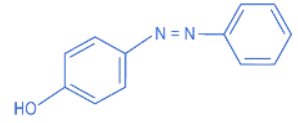
Ans. a
B.S.C (Benzene sulphonyl chloride) is known as Hinsberg Reagent.
Q.9. When enthanol is heated with conc. H2SO4, a gas is produced. The compound formed, when this gas is treated with cold dilute aqueous solution of Baeyer's reagent, is (JEE Main 2022)
(a) formaldehyde
(b) formic acid
(c) glycol
(d) ethanoic acid
Ans. c
Q.10. A compound 'X' is acidic and it is soluble in NaOH solution, but insoluble in NaHCO3 solution. Compound 'X' also gives violet colour with neutral FeCl3 solution. The compound 'X' is: (JEE Main 2022)
(a)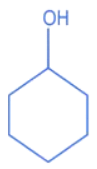
(b)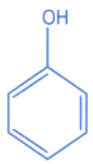
(c)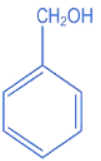
(d)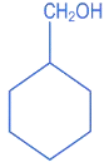
Ans. c
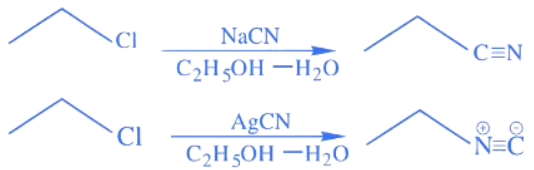
Q.11. Considering the reactions, the compound 'A' and compound 'B' respectively are: (JEE Main 2022)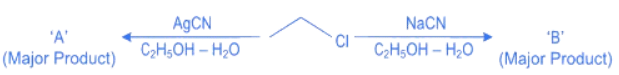 (a)
(a)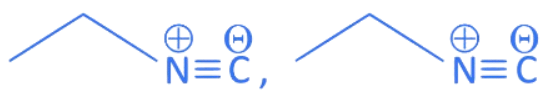
(b)
(c)
(d)
Ans. c
In NaCN; carbon is more nucleophilic atom.
Whereas in AgCN; Ag – C has covalent bond.
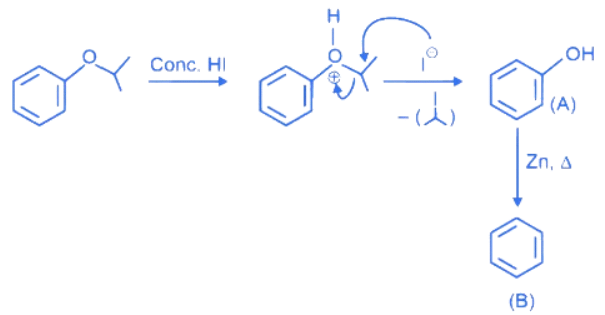
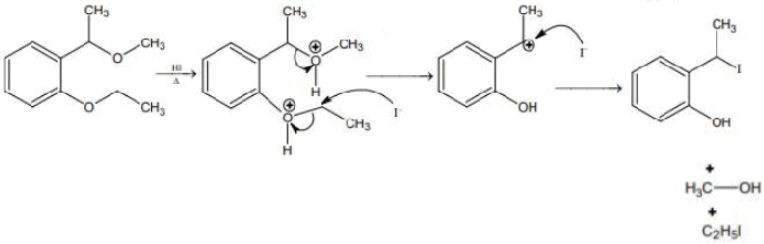
Q.12. Compound I is heated with Conc. HI to give a hydroxy compound A which is further heated with Zn dust to give compound B. Identify A and B. (JEE Main 2022)  (a)
(a)
(b)
(c)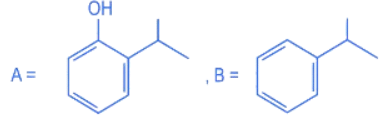
(d)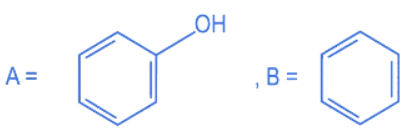
Ans. d
Q.13. The major product in the given reaction is (JEE Main 2022) 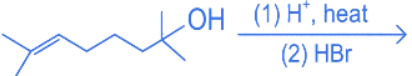
(a)
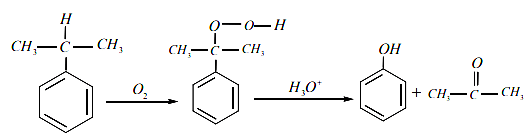
(b)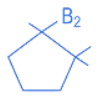
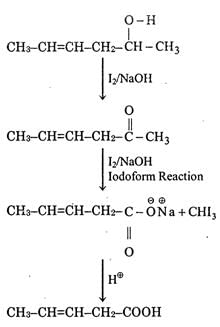
(c)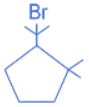
(d)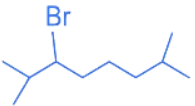
Ans. c
Q.14. Identify the major products A and B for the below given reaction sequence. (JEE Main 2022) 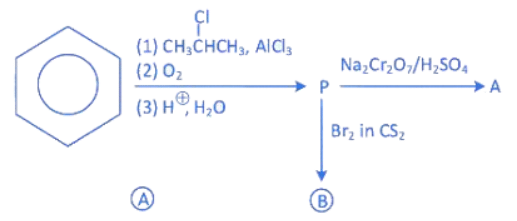 (a)
(a)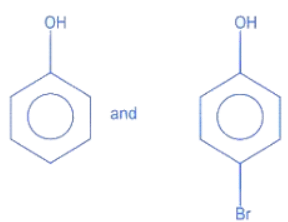
(b)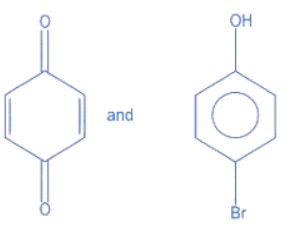
(c)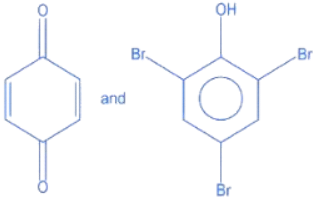
(d)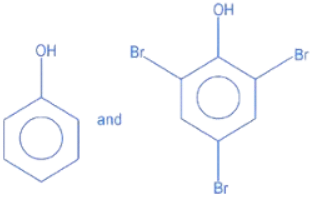
Ans. c
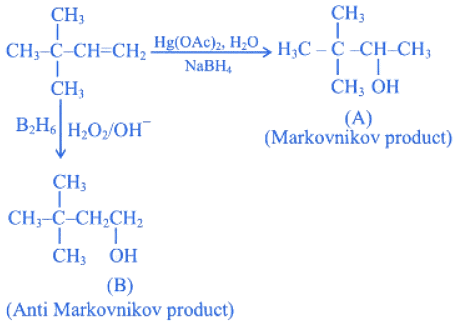
Q.15. Choose the correct option for the following reactions. (JEE Main 2022) 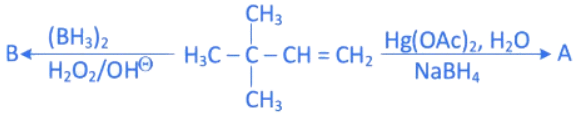 (a) 'A' and 'B' are both Markovnikov addition products.
(a) 'A' and 'B' are both Markovnikov addition products.
(b) 'A' is Markovnikov product and 'B' is anti-Markovnikov product.
(c) 'A' and 'B' are both anti-Markovnikov products.
(d) 'B' is Markovnikov and 'A' is anti-Markovnikov product.
Ans. b
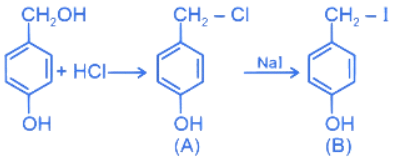
Q.16.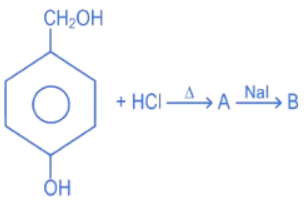 In the above reaction product B is: (JEE Main 2022)
In the above reaction product B is: (JEE Main 2022)
(a)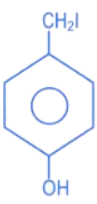
(b)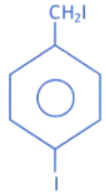
(c)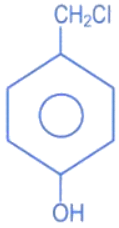
(d)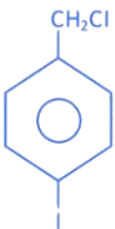
Ans. a
Product B is 4-iodomethylphenol.
Q.17. Which reactant will give the following alcohol on reaction with one mole of phenyl magnesium bromide (PhMgBr) followed by acidic hydrolysis? (JEE Main 2022)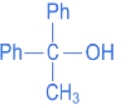 (a) CH3 − C ≡ N
(a) CH3 − C ≡ N
(b) Ph − C ≡ N
(c)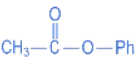
(d)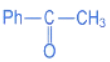
Ans. d
Q.18. The difference in the reaction of phenol with bromine in chloroform and bromine in water medium is due to: (JEE Main 2022)
(a) Hyperconjugation in substrate
(b) Polarity of solvent
(c) Free radical formation
(d) Electromeric effect the substrate
Ans. b
Phenol gives different products with bromine in chloroform and water medium due to the polarity difference between chloroform and water acting as solvent.
Q.19. What is the major product of the following reaction? (JEE Main 2022)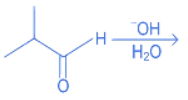
(a)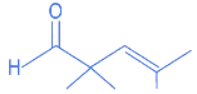
(b)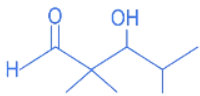
(c)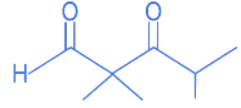
(d)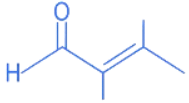
Ans. b
Q.20. The reagent neutral ferric chloride is used to detect the presence of ______________ (JEE Main 2022)
(a) sulphide ion and alcoholic −OH group.
(b) acetate ion and phenolic −OH group.
(c) sulphide ion and phenolic −OH group.
(d) acetate ion and alcoholic −OH group.
Ans. b
Acetate ions gives deep red colour ppt on reaction with netural ferric chloride solution.
Phenol reacts with freshly prepared ferric chloride solution and gives violet colour complex.
Q.21.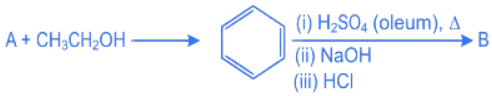 Consider the above reaction sequence. Identify the component A and component B: (JEE Main 2022)
Consider the above reaction sequence. Identify the component A and component B: (JEE Main 2022)
(a)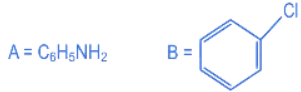
(b)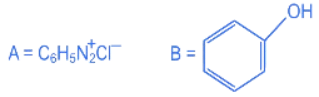
(c)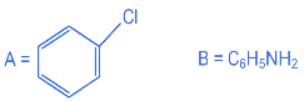
(d)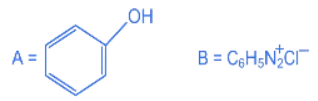
Ans. b
Q.22. Given below are two statements:
Statement I: Phenols are weakly acidic.
Statement II: Therefore they are freely soluble in NaOH solution and are weaker acids than alcohols and water.
Choose the most appropriate option: (JEE Main 2022)
(a) Both Statement I and Statement II are correct.
(b) Both Statement I and Statement II are incorrect.
(c) Statement I is correct but Statement II is incorrect.
(d) Statement I is incorrect but Statement II is correct.
Ans. c
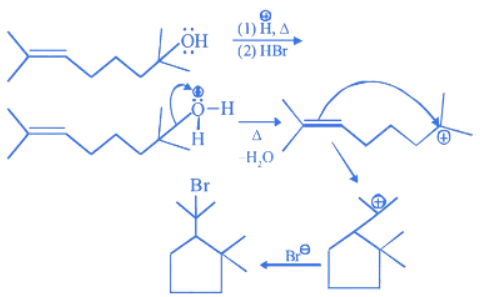
Q.23. The major product (P) of the given reaction is
(where, Me is −CH3) (JEE Main 2022)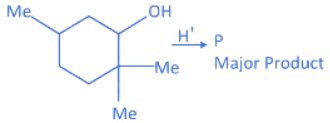 (a)
(a)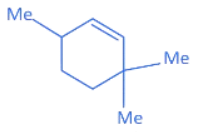
(b)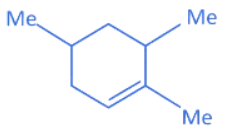
(c)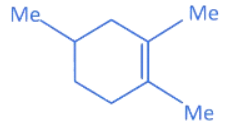
(d)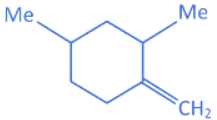
Ans. c
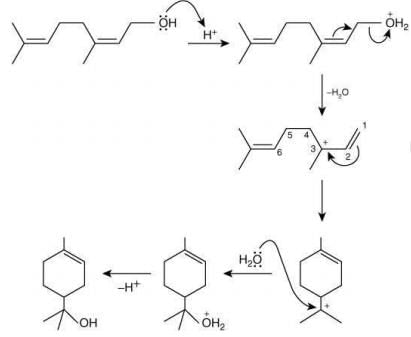
(1) In compound A, positive charge on O atom is not stable that is why O⊕H2 get's removed.
(2) In Carbocation (B), two α-H present and after 1, 2-methyl shift number of α-H becomes six so it becomes more stable as far carbocation when number of α-H increases stability increases.
(3) Among product D, E and F, D is major product as in case of alkene most stable, alkene are those which have more α-H. And every reactant produce that product which is more stable.
Q.24. The major product in the following reaction (JEE Main 2022)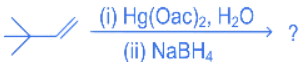 (a)
(a)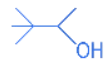
(b)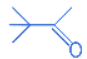
(c)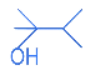
(d)
Ans. a
Q.25. The major product formed in the following reaction, is (JEE Main 2022)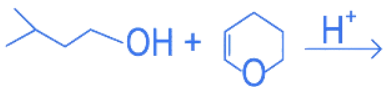 (a)
(a)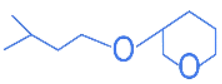
(b)
(c)
(d)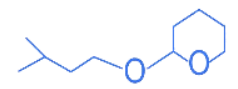
Ans. d
Q.26. In the given reaction 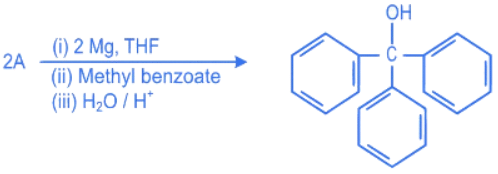 'A' can be (JEE Main 2022)
'A' can be (JEE Main 2022)
(a) benzyl bromide
(b) bromobenzene
(c) cyclohexyl bromide
(d) methyl bromide
Ans. b
Q.27. The intermediate X, in the reaction: (JEE Main 2022) 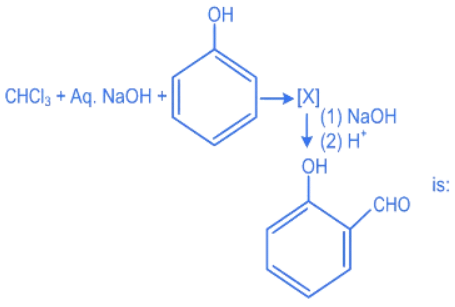 (a)
(a)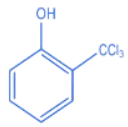
(b)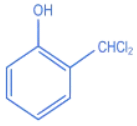
(c)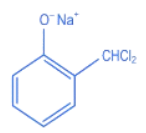
(d)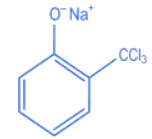
Ans. c
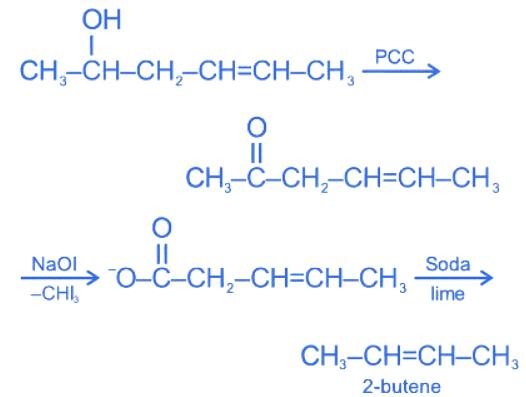
Q.28. Hex-4-ene-2-ol on treatment with PCC gives 'A'. 'A' on reaction with sodium hypoiodite gives 'B', which on further heating with soda lime gives 'C'. The compound 'C' is: (JEE Main 2022)
(a) 2-pentene
(b) proponaldehyde
(c) 2-butene
(d) 4-methylpent-2-ene
Ans. c
Q.29. The major product of the following reaction is: (JEE Main 2021) 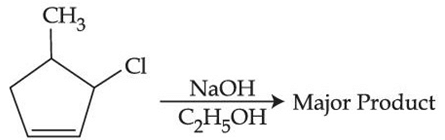 (a)
(a)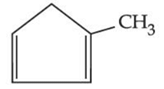
(b)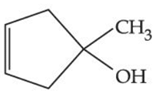
(c)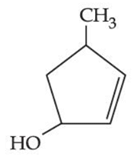
(d)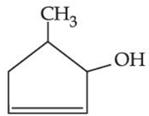
Ans. d
NaOH + EtOH is known as alcoholic NaOH, so it give E2 reaction with given alkyl halide.
Q.30. The structures A and B formed in the following reaction are: [Ph = −C6H5] (JEE Main 2021)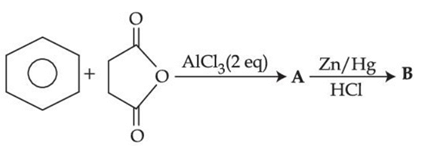 (a)
(a)
(b)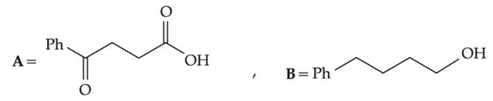
(c)
(d)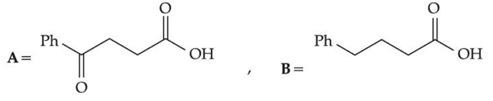
Ans. d
Q.31. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): Treatment of bromine water with propene yields 1-bromopropan-2-ol.
Reason (R): Attack of water on bromonium ion follows Markovnikov rule and results in 1-bromopropan-2-ol.
In the light of the above statements, choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) Both (A) and (R) are true but (R) is not the correct explanation of (A)
(b) (A) is false but (R) is true
(c) Both (A) and (R) are true and (R) is the correct explanation of (A)
(d) (A) is true but (R) is false
Ans. c
Its IUPAC name 1-bromopropan-2-ol
A and R are true and (R) is the correct explanation of (A).
Q.32. The major product formed in the following reaction is: (JEE Main 2021) 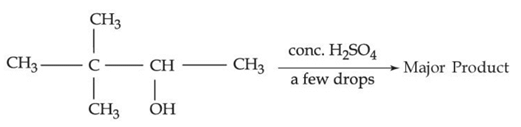 (a)
(a)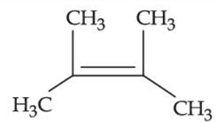
(b)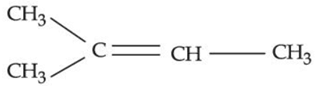
(c)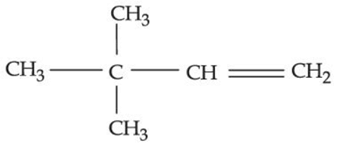
(d)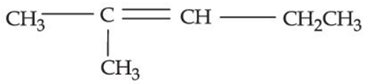
Ans. a
Q.33. The major product of the following reaction, if it occurs by SN2 mechanism is: (JEE Main 2021)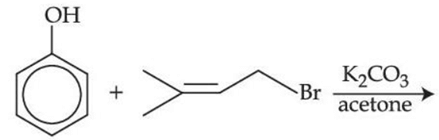 (a)
(a)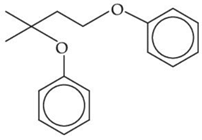
(b)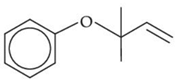
(c)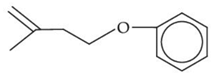
(d)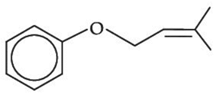
Ans. d
Q.34. Given below are two statements:
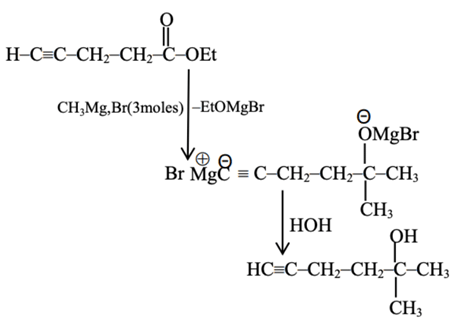
Statement I: Ethyl pent-4-yn-oate on reaction with CH3MgBr gives a 3∘-alcohol.
Statement II: In this reaction one mole of ethyl pent-4-yn-oate utilizes two moles of CH3MgBr.
In the light of the above statements, choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) Both Statement I and Statement II are false.
(b) Statement I is false but Statement II is true.
(c) Statement I is true but Statement II is false.
(d) Both Statement I and Statement II are true.
Ans. c
Statement I is true
But it consume 3 moles of G R
So Statement II is false.
Q.35. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
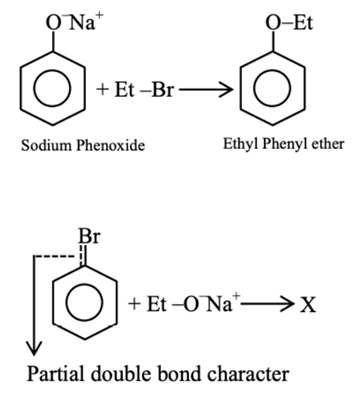
Assertion (A): Synthesis of ethyl phenyl ether may be achieved by Williamson synthesis.
Reason (R): Reaction of bromobenzene with sodium ethoxide yields ethyl phenyl ether.
In the light of the above statements, choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) Both (A) and (R) are correct and (R) is the correct explanation of (A)
(b) (A) is correct but (R) is not correct
(c) (A) is not correct but (R) is correct
(d) Both (A) and (R) are correct but (R) is not the correct explanation of (A)
Ans. b
Q.36. The major product of the following reaction is: (JEE Main 2021) 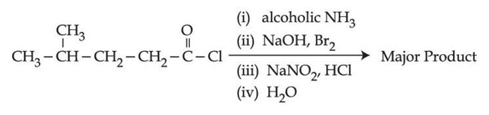 (a)
(a)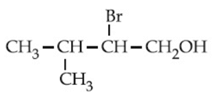
(b)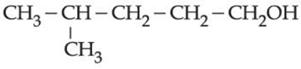
(c)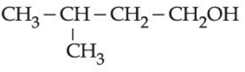
(d)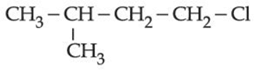
Ans. c
Q.37. Which one of the following phenols does not give colour when condensed with phthalic anhydride in presence of conc. H2SO4? (JEE Main 2021)
(a)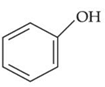
(b)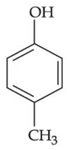
(c)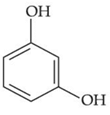
(d)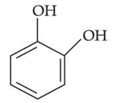
Ans. b
Only p-methyl, phenol does not give any colour with phthalic anhydroxide with cons. H2SO4.
Q.38. The correct options for the products A and B of the following reactions are: (JEE Main 2021) 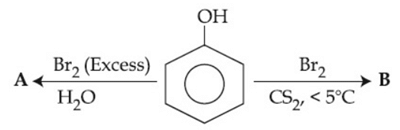 (a)
(a)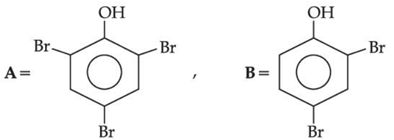
(b)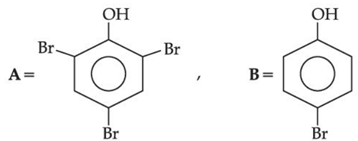
(c)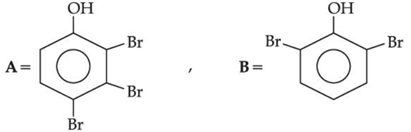
(d)
Ans. b
Q.39.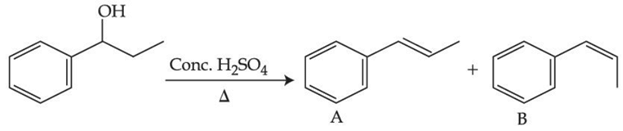
Consider the above reaction, and choose the correct statement: (JEE Main 2021)
(a) The reaction is not possible in acidic medium
(b) Both compounds A and B are formed equally
(c) Compound A will be the major product
(d) Compound B will be the major product
Ans. c
Q.40.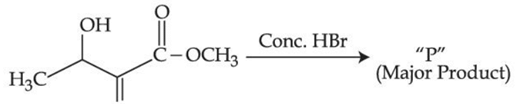 Consider the above reaction, the major product "P" formed is: (JEE Main 2021)
Consider the above reaction, the major product "P" formed is: (JEE Main 2021)
(a)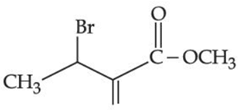
(b)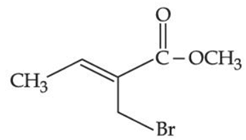
(c)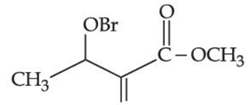
(d)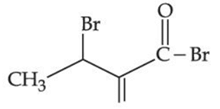
Ans. b
Q.41.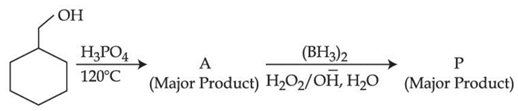 Consider the above reaction and identify the Product P: (JEE Main 2021)
Consider the above reaction and identify the Product P: (JEE Main 2021)
(a)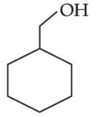
(b)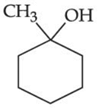
(c)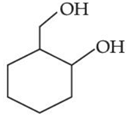
(d)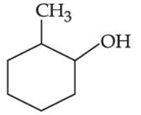
Ans. d
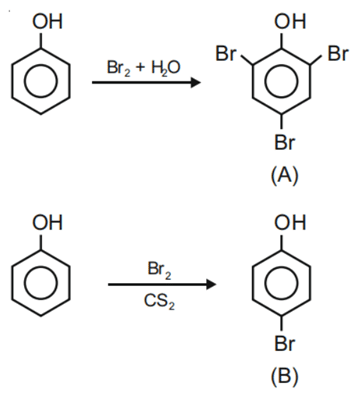
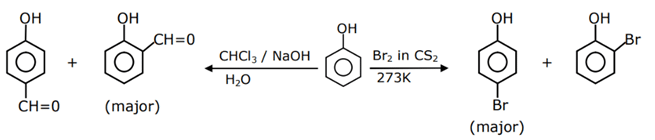
Q.42.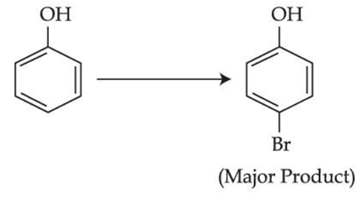 The given reaction can occur in the presence of:
The given reaction can occur in the presence of:
(a) Bromine water
(b) Br2 in CS2, 273 K
(c) Br2/FeBr3
(d) Br2 in CHCl3, 273 K
Choose the correct answer from the options given below: (JEE Main 2021)
(a) (b) and (d) only
(b) (a) and (c) only
(c) (b), (c) and (d) only
(d) (a), (b) and (d) only
Ans. c
Bromine water gives tribromo products, other gives monobromo products in which para is major product.
Q.43.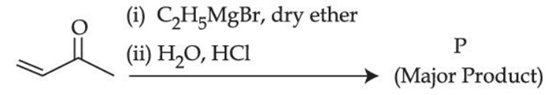 Consider the above reaction, the major product 'P' is: (JEE Main 2021)
Consider the above reaction, the major product 'P' is: (JEE Main 2021)
(a)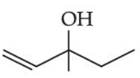
(b)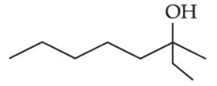
(c)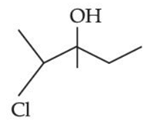
(d)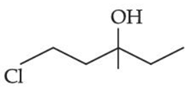
Ans. c
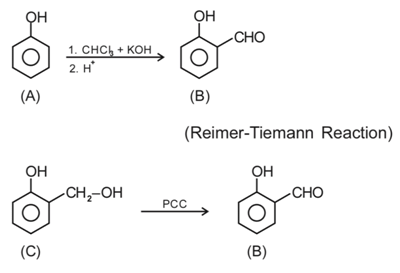
Q.44. An organic compound A (C6H6O) gives dark green colouration with ferric chloride. On treatment with CHCl3 and KOH, followed by acidification gives compound B. Compound B can also be obtained from compound C on reaction with pyridinium chlorochromate (PCC). Identify A, B and C. (JEE Main 2021)
(a)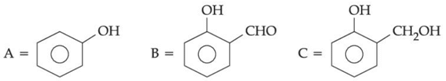
(b)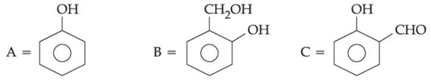
(c)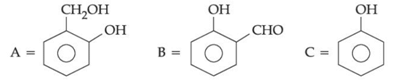
(d)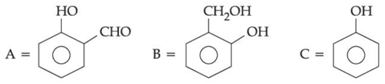
Ans. a
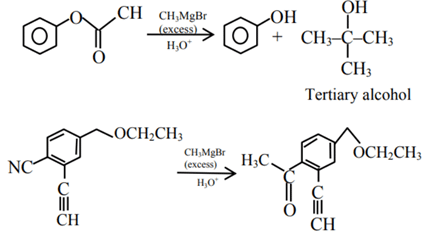
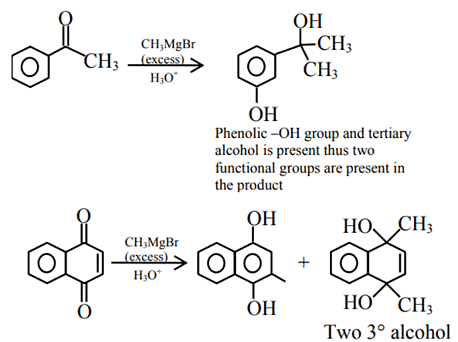
Q.45. Which of the following compounds will provide a tertiary alcohol or reaction with excess of CH3MgBr followed by hydrolysis? (JEE Main 2021)
(a)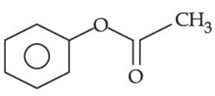
(b)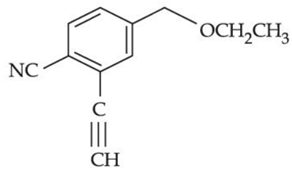
(c)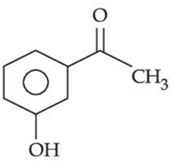
(d)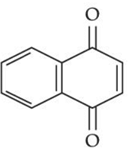
Ans. a
Since, the given question is single correct choice the best appropriate option is (A).
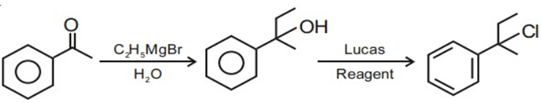
Q.46. Reaction of Grignard reagent, C2H5MgBr with C8H8O followed by hydrolysis gives compound "A" which reacts instantly with Lucas reagent to give compound B, C10H13Cl. The Compound B is: (JEE Main 2021)
(a)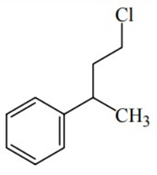
(b)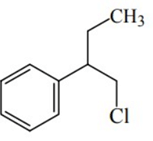
(c)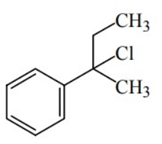
(d)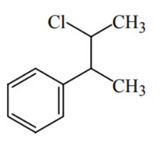
Ans. c
Reaction occur through formation of carbocation.
Q.47. Given below are two statements:
Statement I: 2-methylbutane on oxidation with KMnO4 gives 2-methylbutan-2-ol.
Statement II: n-alkanes can be easily oxidised to corresponding alcohols with KMnO4.
Choose the correct option: (JEE Main 2021)
(a) Both statement I and statement II are incorrect
(b) Both statement I and statement II are correct
(c) Statement I is correct but statement II is incorrect
(d) Statement I is incorrect but statement II is correct
Ans. c
Alkanes having tertiary H can be oxidized to corresponding alcohols by KMnO4.
whereas ordinary alkanes resist oxidation.
Q.48. Identify A in the given reaction. (JEE Main 2021)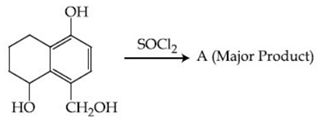 (a)
(a)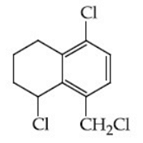
(b)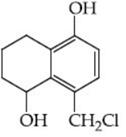
(c)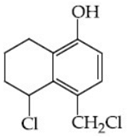
(d)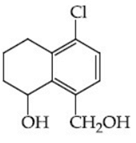
Ans. c
Q.49. Ceric ammonium nitrate and CHCl3/alc. KOH are used for the identification of functional groups present in __________ and _______ respectively. (JEE Main 2021)
(a) amine, alcohol
(b) alcohol, phenol
(c) alcohol, amine
(d) amine, phenol
Ans. c
Alcohol give positive test with ceric ammonium nitrate and primary amines gives carbyl amine test with CHCl3, KOH.
Q.50. B reacts with Hydroxyl amine but does not give Tollen's test. Identify A and B. (JEE Main 2021)
B reacts with Hydroxyl amine but does not give Tollen's test. Identify A and B. (JEE Main 2021)
(a) 1, 1-Dichlorobutane and Butanal
(b) 2, 2-Dichlorobutane and Butan-2-one
(c) 1, 1-Dichlorobutane and 2-Butanone
(d) 2, 2-Dichlorobutane and Butanal
Ans. b
Compound 'B' does not gives Tollen's test due to presence of kenotic group but react with hydroxyl amine.
Q.51. Given below are two statements:
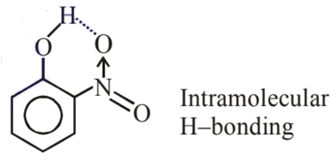
Statement I: o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement II: o-Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below: (JEE Main 2021)
(a) Both Statement I and Statement II are true
(b) Statement I is true but Statement II is false
(c) Statement I is false but Statement II is true
(d) Both Statement I and Statement II are false
Ans. b
So it is more volatile due to intramolecular H-bonding.
Melting point depends on packing efficiency not on H-bonding thus statement II is false.
Q.52. Identify the major products A and B respectively in the following reactions of phenol: (JEE Main 2021)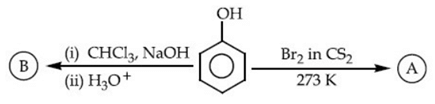 (a)
(a)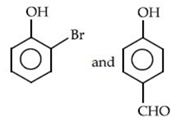
(b)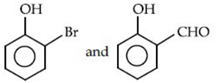
(c)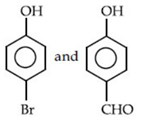
(d)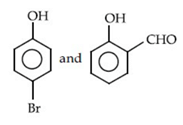
Ans. d
Q.53. What is 'X' in the given reaction? (JEE Main 2021)
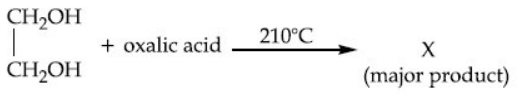 (a)
(a)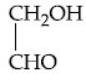
(b)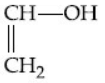
(c)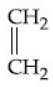
(d)
Ans. c
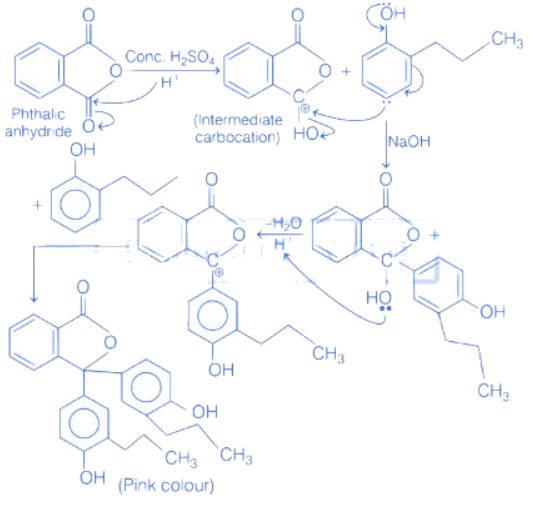
Q.54. Which of the following compound gives pink colour on reaction with phthalic anhydride in conc. H2SO4 followed by treatment with NaOH? (JEE Main 2021)
(a)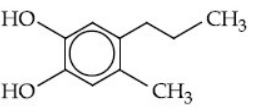
(b)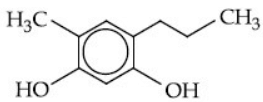
(c)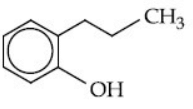
(d)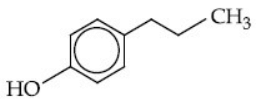
Ans. c
Firstly phthalic anhydride in presence of conc. H2SO4 undergoes protonation to give an intermediate carbocation. This carbocation reacts with 2-propylphenol in presence of NaOH to give pink colour compound.
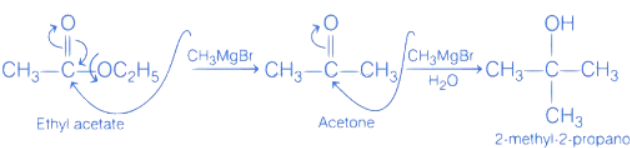
Q.55. To synthesize 1.0 mole of 2-methylpropan-2-ol from Ethylethanoate ___________ equivalents of CH3MgBr Reagent will be required. (Integer value) (JEE Main 2021)
Ans. 2
2 moles of CH3MgBr reagent will be used. Chemical reaction is as follows
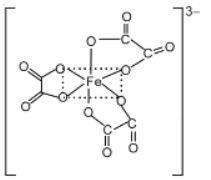
Q.56. On complete reaction of FeCl3 with oxalic acid in aqueous solution containing KOH, resulted in the formation of product A. The secondary valency of Fe in the product A is __________. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 6
FeCl3 + 3H2C2O4 + 6KOH → K3[Fe(C2O4)3] + 3KCl + 6H2O
Coordination number = 6
Secondary valency is 6
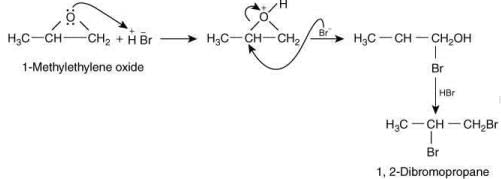
Q.57. 1-Methyl ethylene oxide when treated with an excess of HBr produces (2020)
(a) 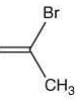
(b) 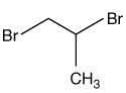
(c) 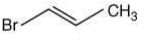
(d) 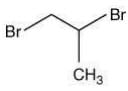
Ans. b
The reaction involved is
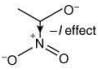
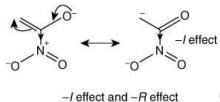
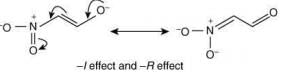
Q.58. The correct order of stability for the following alkoxides is (2020)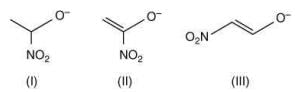 (a) (II) > (I) > (III)
(a) (II) > (I) > (III)
(b) (III) > (II) > (I)
(c) (III) > (I) > (II)
(d) (II) > (III) > (I)
Ans. b
The stability of the following alkoxides depends upon the dispersion of the negative charge on oxygen atom in the molecule.
Alkoxide (I) is stabilized by –I effect of -NO2 group.
Alkoxide (II) is stabilized by –I effect of -NO2 group and –R effect of double bond.
Alkoxide (III) is stabilized by –I effect of -NO2 group and –R effect (long distance conjugation).
Thus, the correct order of stability of alkoxide is: (III) > (II) > (I).
Q.59. In the following reaction sequence, structures of A and B, respectively will be (2020)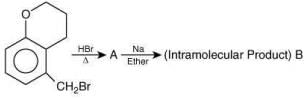 (a)
(a) 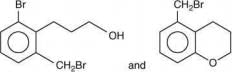
(b) 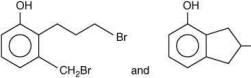
(c) 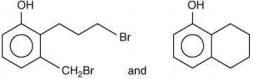
(d) 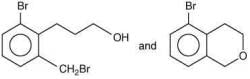
Ans. c
The reaction involved is
Q.60. Arrange the following compounds in increasing order of C–OH bond length; methanol, phenol, pethoxyphenol (2020)
(a) Methanol < p-ethoxyphenol < phenol
(b) Phenol < methanol < p-ethoxyphenol
(c) Phenol < p-ethoxyphenol < methanol
(d) Methanol < phenol < p-ethoxyphenol
Ans. c
Due to the delocalization of lone pair electrons of oxygen into the benzene ring, thus, the C-OH bond in phenol have partial double bond character. In p-ethoxyphenol, the lone pair of electrons also delocalized in the ring but due to the presence of ethyl group attached to oxygen atom, so, the C-OH bond in pethoxyphenol have less than partial double bond character. In methanol, the bond between C-OH have single bond. Hence, the increasing order of C-OH bond is: phenol < p-ethoxyphenol < methanol.
Q.61. The major product of the following reaction is (2020)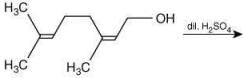 (a)
(a) 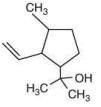
(b) 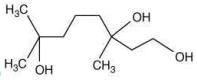
(c) 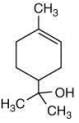
(d) 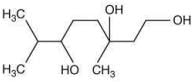
Ans. c
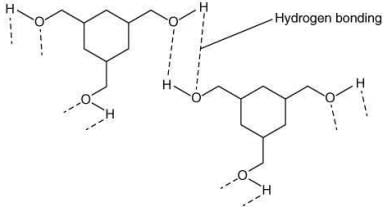
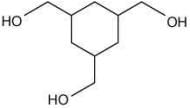
Q.62. Among the compounds A and B with molecular formula C9H18O3, A is having higher boiling point the B. The possible structures of A and B are (2020)
(a) 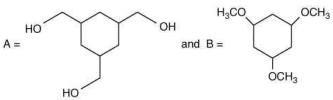
(b) 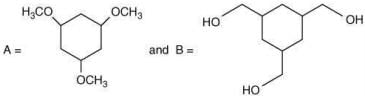
(c) 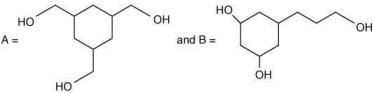
(d) 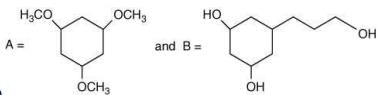
Ans. a
The compound that will show more hydrogen bonding will have high boiling point. Compound (I) have higher boiling point than compound (II), because compound (I) show hydrogen bonding.
Thus, the possible structure for I is:
The possible structure for II is:
Q.63. The major product of the following reaction is: (2019)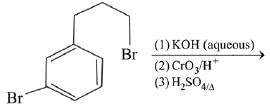 (a)
(a) 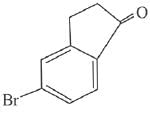
(b) 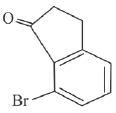
(c) 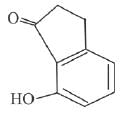
(d) 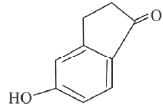
Ans. a
Q.64. The compounds A and B in the following reaction are, respectively: (2019)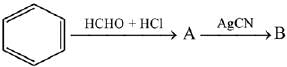 (a) A = Benzyl alcohol, B = Benzyl cyanide
(a) A = Benzyl alcohol, B = Benzyl cyanide
(b) A =Benzyl chloride, B = Benzyl cyanide
(c) A = Benzyl alcohol, B = Benzyl isocyanide
(d) A = Benzyl chloride, B = Benzyl isocyanide
Ans. d
Q.65. The major product obtained in the following reaction is: (2019)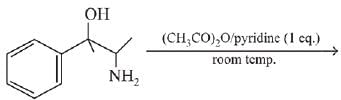 (a)
(a) 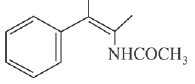
(b) 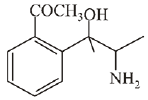
(c) 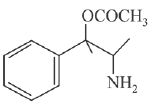
(d) 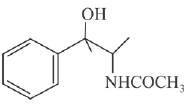
Ans. d
Reaction involved:
Q.66. The products formed in the reaction of cumene with O2 followed by treatment with dil. HCl are: (2019)
(a) 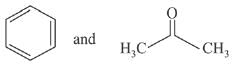
(b) 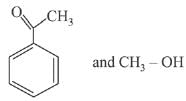
(c) 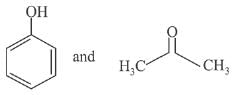
(d) 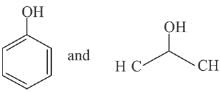
Ans. c
Reaction involved:
Q.67. Which is the most suitable reagent for the following transformation? (2019)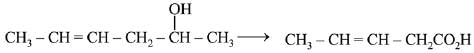 (a) Tollen’s reagent
(a) Tollen’s reagent
(b) I2/NaOH
(c) CrO2Cl2/CS2
(d) alkaline KMnO4
Ans. b
The most suitable reagent for the given reaction is I2/NaOH (Iodoform reaction).
Q.68. Which of the following compounds reacts with ethyl-magnesium bromide and also decolourizes bromine water solution? (2019)
(a) 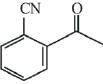
(b) 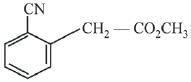
(c) 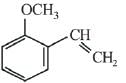
(d) 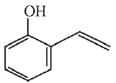
Ans. d
Q.69. The major product in the following conversion is: (2019)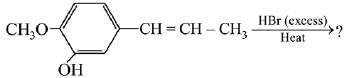 (a)
(a) 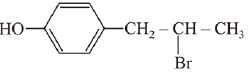
(b) 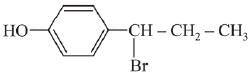
(c) 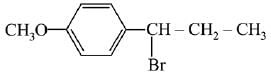
(d) 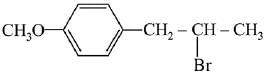
Ans. b
Q.70. The major product of the following reaction is: (2019)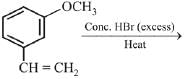 (a)
(a) 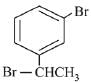
(b) 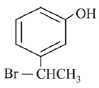
(c) 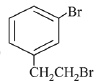
(d) 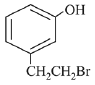
Ans. a
Q.71. The organic compound that gives following qualitative analysis is: (2019)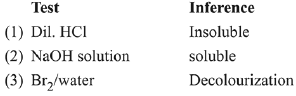
(a) 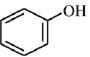
(b) 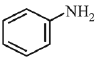
(c) 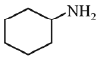
(d) 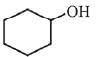
Ans. a
Q.72. The major product of the following reaction is: (2019)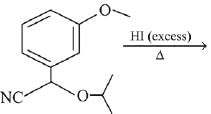 (a)
(a) 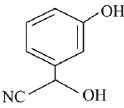
(b) 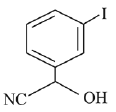
(c) 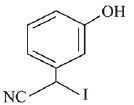
(d) 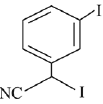
Ans. c
Q.73. What will be the major product when m-cresol is reacted with propargyl bromide (HC ≡ C — CH2Br) in presence of K2CO3 in acetone? (2019)
(a) 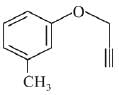
(b) 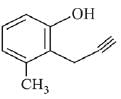
(c) 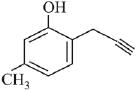
(d) 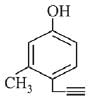
Ans. a
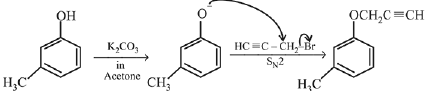
Q.74. Phenol reacts with methyl chloroformate in the presence of NaOH to form product A. A reacts with Br2 to form product B. A and B are respectively: (2018)
(a) 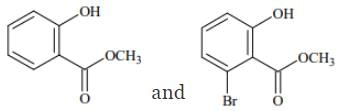
(b) 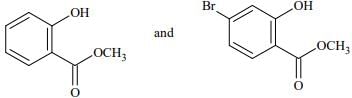
(c) 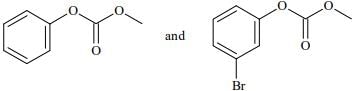
(d) 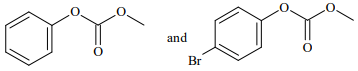
Ans. d
Q.75. The major product formed in the following reaction is: (2018)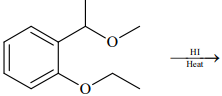 (a)
(a) 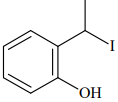
(b) 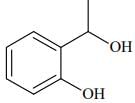
(c) 
(d) 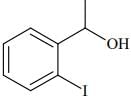
Ans. a
Q.76. Phenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces: (2018)
(a) 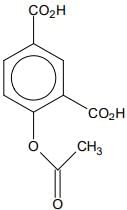
(b) 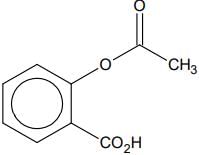
(c) 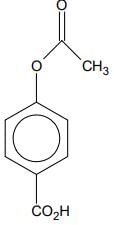
(d) 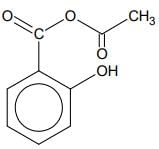
Ans. b
Q.77. The major product of the following reaction is: (2018)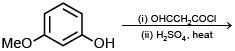 (a)
(a) 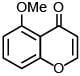
(b) 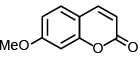
(c) 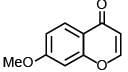
(d) 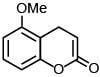
Ans. a
Q.78. The major product of the following reaction is: (2017)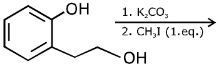 (a)
(a) 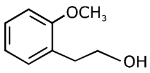
(b) 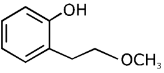
(c) 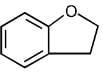
(d) 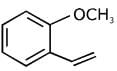
Ans. a
Q.79. The increasing order of the boiling points for the following compounds is: (2017)
(i) C2H5OH
(ii) C2H5Cl
(iii) C2H5CH3
(iv) C2H5OCH3
(a) (IV) < (III) < (I) < (II)
(b) (III) < (II) < (I) < (IV)
(c) (III) < (IV) < (II) < (I)
(d) (II) < (III) < (IV) < (I)
Ans. c
B.P. ∝ dipole moment∝ H-bonding
C2H5CH3 < C2H5OCH3 < C2H5Cl < C2H5OH
Q.80. In the following reaction sequence: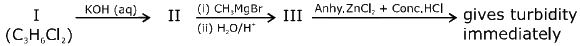 The compound I is: (2017)
The compound I is: (2017)
(a) 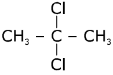
(b) 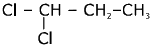
(c) 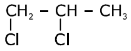
(d)
Ans. a
Q.81. The gas evolved on heating CH3MgBr in methanol is: (2016)
(a) Propane
(b) Ethane
(c) HBr
(d) Methane
Ans. d
CH3MgBr + CH3–OH → (CH3O)MgBr + CH4↑ gas.
Q.82. Bouveault-Blanc reduction reaction involves: (2016)
(a) Reduction of an anhydride with LiAlH4.
(b) Reduction of an ester with Na/C2H5OH.
(c) Reduction of a carbonyl compound with Na/Hg and HCl
(d) Reduction of an acyl halide with H2/Pd.
Ans. b
Reduction using Na in ethyl alcohol is called Bouveault-Blanc reduction.
FAQs on JEE Main Previous Year Questions (2016- 2025): Alcohols, Phenols & Ethers
| 1. What are the main differences between alcohols, phenols, and ethers? |  |
| 2. How are alcohols classified? |  |
| 3. What are some common methods for the synthesis of alcohols? |  |
| 4. What is the significance of phenols in organic chemistry? |  |
| 5. How do ethers react with other compounds? |  |