NCERT Summary: Summary of Physics- 1 | Science & Technology for UPSC CSE PDF Download
| Table of contents |

|
| Atomic Physics |

|
| Radioactivity |

|
| Heat |

|
| Latent Heat of Fusion and Vaporization Chart |

|
Atomic Physics
An atom is the smallest particle of the element that can exist independently and retain all its chemical properties.
1. Dalton’s atomic theory: It suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory.
2. Thomson's Atomic Theory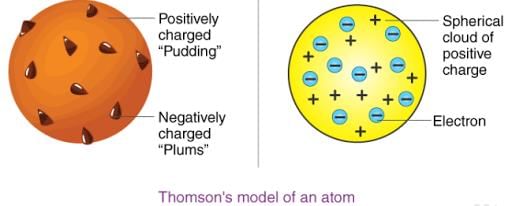
Thomson proposed that:
(i) An atom consists of a positively charged sphere and the electrons are embedded in it.
(ii) The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
3. Rutherford’s model of the atom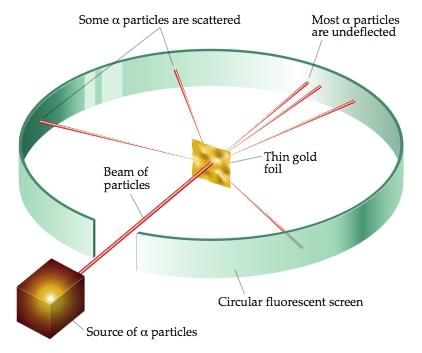 Rutherford's Alpha-particle Scattering Experiment
Rutherford's Alpha-particle Scattering Experiment
- Rutherford’s alpha-particle scattering experiment led to the discovery of the atomic nucleus.
- Rutherford’s model of the atom proposed that a very tiny nucleus is present inside the atom and electrons revolve around this nucleus.
- The stability of the atom could not be explained by this model.
4. Bohr's Model: Neils Bohr’s model of the atom was more successful. He proposed that electrons are distributed in different shells with discrete energy around the nucleus. If the atomic shells are complete, then the atom will be stable and less reactive.
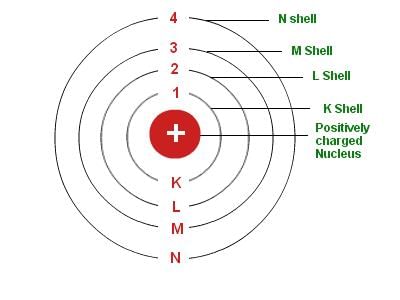 Bohr's Model
Bohr's Model
5. James Chadwick Discovery: J. Chadwick discovered the presence of neutrons in the nucleus of an atom.
The three sub-atomic particles of an atom are:
(i) Electrons
(ii) Protons
(iii) Neutrons
Electrons are negatively charged, protons are positively charged and neutrons have no charges. The mass of an electron is about 1/2000 times the mass of a hydrogen atom. The mass of a proton and a neutron is taken as one unit each.
- We know that protons are present in the nucleus of an atom. It is the number of protons of an atom, which determines its atomic number. It is denoted by ‘Z’. All atoms of an element have the same atomic number, Z. In fact, elements are defined by the number of protons they possess.
- The mass of an atom is practically due to protons and neutrons alone. These are present in the nucleus of an atom. Hence protons and neutrons are also called nucleons. Therefore, the mass of an atom resides in its nucleus.
- Isotopes are atoms of the same element, which have different mass numbers.
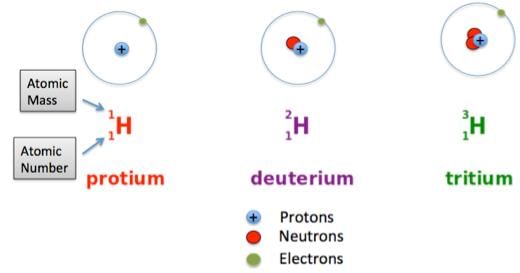
- Isobars are atoms having the same mass number but different atomic numbers.
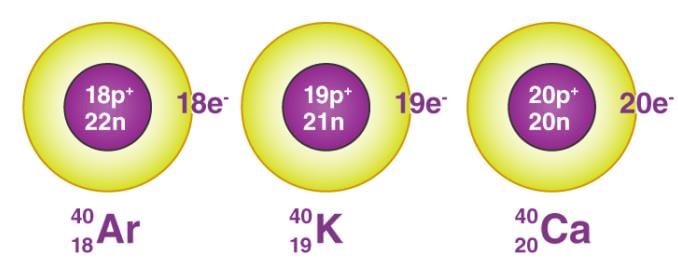 Isobars
Isobars
Nuclear force
- To bind a nucleus together there must be a strong attractive force of a totally different kind. It must be strong enough to overcome the repulsion between the (positively charged) protons and to bind both protons and neutrons into the tiny nuclear volume. This force is called the Nuclear Force.
- The nuclear force is much stronger than the Coulomb force acting between charges or the gravitational forces between masses. The nuclear force between neutron-neutron, proton-neutron and proton-proton is approximately the same. The nuclear force does not depend on the electric charge.
Radioactivity
- Radioactivity occurs when an atomic nucleus breaks down into smaller particles. There are three types of nuclear radiation: Alpha, Beta, and Gamma. Alpha particles are positively charged, beta particles are negatively charged, and gamma particles have no charge. The radiations also have increased levels of energy, first Alpha, then Beta, and finally, Gamma, which is the most energetic of all these. Alpha and Beta are particles, but Gamma is a wave.
- When a radioactive nucleus changes, the remaining nucleus (and atom) is not the same as it was. It changes its identity. The term half-life describes the time it takes for half of the atoms in a sample to change, and half to remain the same.
- There is even a radioactive isotope of carbon, carbon-14. Normal carbon is carbon-12. C-14 has two extra neutrons and a half-life of 5730 years. Scientists use C-14 in a process called carbon dating. This process is not when two carbon atoms go out to the mall one night. Carbon dating is when scientists try to measure the age of very old substances. There are very small amounts of C-14 in the atmosphere. Every living thing has some C-14 in it. Scientists measure the number of C-14 in the things they dig up to estimate how old they are. They rely on the half-life of 5730 years to date the object.
Fission and Fusion Reactions
- Fission is the splitting of an atom. Not all atoms will go through fission, as a matter of fact, very few do under normal circumstances.
- In a nuclear reaction, scientists shoot a whole bunch of neutrons at uranium- 235 atoms. When one neutron hits the nucleus, the uranium becomes U-236. When it becomes 236, the uranium atom wants to split apart. After it splits, it gives off three neutrons and a lot of energy. Those neutrons hit three other U atoms in the area and cause them to become U-236. Each cycle, the reaction gets three times bigger. A reaction that, once started, continues by itself, is called a chain reaction.
- Fusion is the process of two small atomic nuclei coming together to make a larger nucleus that is stable. The simplest nuclei to use are deuterium and tritium (isotopes of hydrogen).
Heat
- Temperature is a relative measure or indication of hotness or coldness.
- Heat is the form of energy transferred between two (or more) systems or a system and its surroundings by virtue of temperature difference. The SI unit of heat energy transferred is expressed in joule (J) while S.I. unit of temperature is Kelvin (K), and °C is a commonly used unit of temperature.
- The thermometer is a device used for measuring temperatures. The two familiar temperature scales are the Fahrenheit temperature scale and the Celsius temperature scale.
The Celsius temperature (Tc) and the Fahrenheit temperature (Tf) are related by:
Tf = (9/5) Tc + 32 - In principle, there is no upper limit to temperature but there is a definite lower limit- the absolute zero. This limiting temperature is 273.16° below zero on the Celsius scale of temperature.
- Clinical thermometer is used to measure our body temperature. The range of this thermometer is from 35°C to 42°C. For other purposes, we use laboratory thermometers. The range of these thermometers is usually from – 10°C to 110°C. The normal temperature of the human body is 37°C.
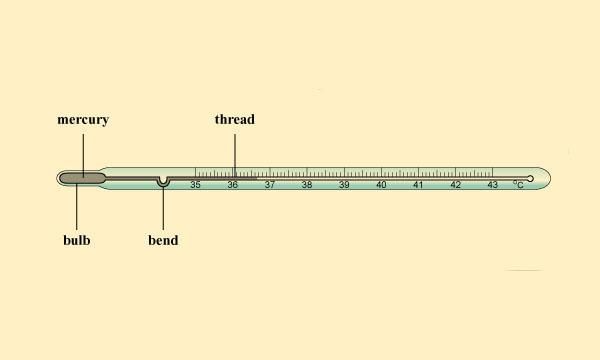 Clinical Thermometer
Clinical Thermometer
- The heat flows from a body at a higher temperature to a body at a lower temperature. There are three ways in which heat can flow from one object to another. These are conduction, convection and radiation.
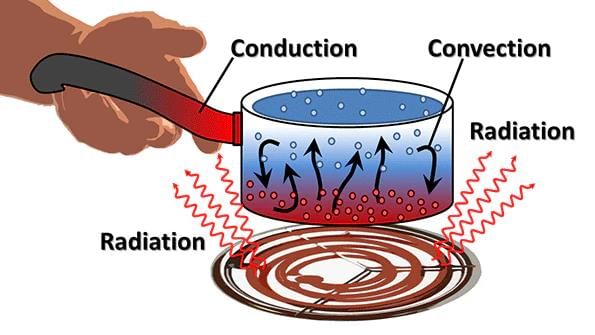 (a) Conduction: The process by which heat is transferred from the hotter end to the colder end of an object is known as conduction. In solids, generally, the heat is transferred by the process of conduction.
(a) Conduction: The process by which heat is transferred from the hotter end to the colder end of an object is known as conduction. In solids, generally, the heat is transferred by the process of conduction.
The materials which allow heat to pass through them easily are conductors of heat. Examples: Aluminium, Iron and Copper.
The materials which do not allow heat to pass through them easily are poor conductors of heat such as plastic and wood. Poor conductors are known as insulators.
(b) Convention: Heat is carried from one place to another by the actual movement of liquid and gases. In liquids and gases, the heat is transferred by convection.
The people living in coastal areas experience an interesting phenomenon. During the day, the land gets heated faster than the water. The air over the land becomes hotter and rises up. The cooler air from the sea rushes in towards the land to take its place. The warm air from the land moves towards the sea to complete the cycle. The air from the sea is called the sea breeze. At night it is exactly the reverse. The water cools down more slowly than the land. So, the cool air from the land moves towards the sea. This is called the land breeze.
(c) Radiation: The transfer of heat by radiation does not require any medium. It can take place whether a medium is present or not.
Dark-coloured objects absorb radiation better than light-coloured objects. That is the reason we feel more comfortable in light-coloured clothes in the summer. Woollen clothes keep us warm during winter. It is so because wool is a poor conductor of heat and it has air trapped in between the fibres. - A change in the temperature of a body causes a change in its dimensions. The increase in the dimensions of a body due to the increase in its temperature is called thermal expansion. The expansion in length is called linear expansion. The expansion in area is called area expansion. The expansion in volume is called volume expansion.
- The amount of heat energy required to raise the temperature of 1g of a substance through 1° is called the specific heat capacity of the substance. The S.I. Unit of specific heat capacity is(J/kg) K. Water has the highest specific heat capacity which is equal to 4200 (J/kg)K.
- The specific heat capacity is the property of the substance which determines the change in the temperature of the substance (undergoing no phase change) when a given quantity of heat is absorbed (or rejected) by it. It is defined as the amount of heat per unit mass absorbed or rejected by the substance to change its temperature by one unit. It depends on the nature of the substance and its temperature.
- The amount of heat energy required to raise the temperature of a given mass of substance through 1° is called heat capacity or thermal capacity of the substance. It’s S.I. Unit is (J/K).
Latent Heat of Fusion and Vaporization Chart
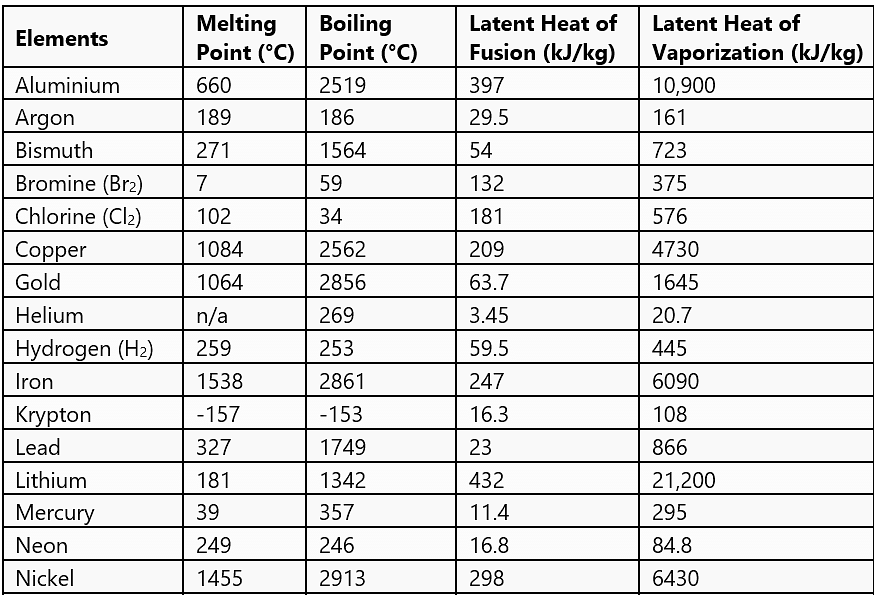
 Normal Phase Change Quantities for Selected Materials
Normal Phase Change Quantities for Selected Materials
- Calorimetry means a measurement of heat. When a body at higher temperature is brought in contact with another body at lower temperature, the heat lost by the hot body is equal to the heat gained by the colder body, provided no heat is allowed to escape to the surroundings. A device in which heat measurement can be made is called a calorimeter.
Change of State
- Matter normally exists in three states: Solid, Liquid, and Gas. A transition from one of these states to another is called a change of state. Two common changes of states are solid to liquid and liquid to gas (and vice versa). These changes can occur when the exchange of heat takes place between the substance and its surroundings.
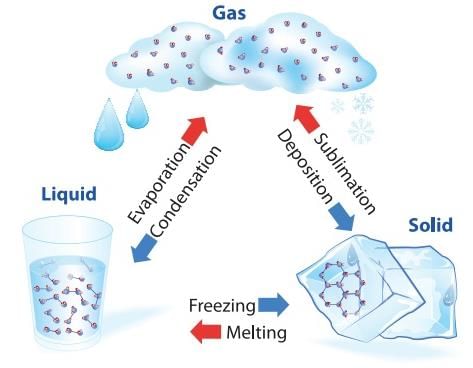 Change of State of Matter
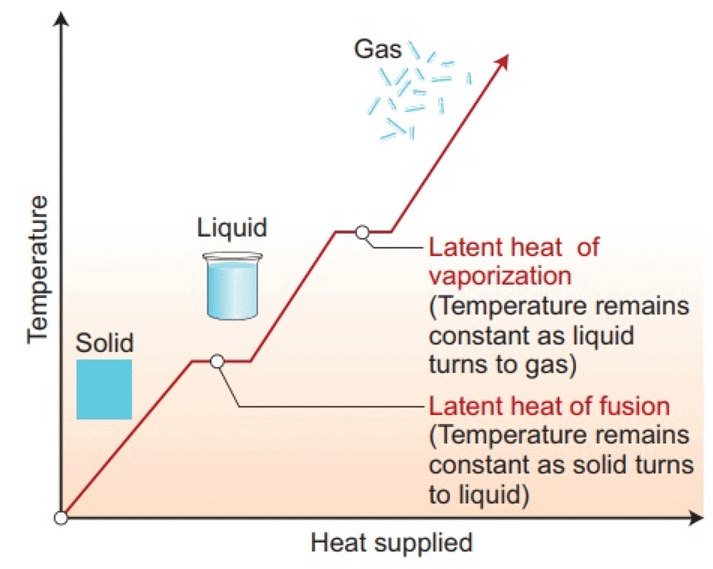
Change of State of Matter - The change of state from solid to liquid is called melting and from liquid to solid is called fusion. It is observed that the temperature remains constant until the entire amount of solid substance melts. That is, both the solid and liquid states of the substance coexist in thermal equilibrium during the change of states from solid to liquid.
- The temperature at which the solid and the liquid states of the substance in thermal equilibrium with each other is called its melting point. It is characteristic of the substance. It also depends on pressure. The melting point of a substance at standard atmospheric pressure is called its normal melting point.
- The change of state from liquid to vapour (or gas) is called vaporisation. It is observed that the temperature remains constant until the entire amount of the liquid is converted into vapour. That is, both the liquid and vapour states of the substance coexist in thermal equilibrium, during the change of state from liquid to vapour.
- The temperature at which the liquid and the vapour states of the substance coexist is called its boiling point. At high altitudes, atmospheric pressure is lower, reducing the boiling point of water as compared to that at sea level. On the other hand, boiling point is increased inside a pressure cooker by increasing the pressure. Hence cooking is faster.
- The boiling point of a substance at standard atmospheric pressure is called its normal boiling point.
- However, all substances do not pass through the three states: Solid-Liquid-Gas. There are certain substances which normally pass from the solid to the vapour state directly and vice versa. The change from solid state to vapour state without passing through the liquid state is called sublimation, and the substance is said to sublime. Dry ice (solid CO2) sublimes, so also iodine. During the sublimation process both the solid and vapour states of a substance coexist in thermal equilibrium.
- A certain amount of heat energy is transferred between a substance and its surroundings when it undergoes a change of state. The amount of heat per unit mass transferred during change of state of the substance is called latent heat of the substance for the process.
- The amount of heat energy supplied to a solid at its melting point, such that it changes into liquid state without any rise in temperature is called latent heat of fusion and that for a liquid-gas state change is called the latent heat of vaporisation.
- Newton’s Law of Cooling says that the rate of cooling of a body is proportional to the excess temperature of the body over the surroundings.
|
90 videos|488 docs|209 tests
|
FAQs on NCERT Summary: Summary of Physics- 1 - Science & Technology for UPSC CSE
| 1. What is radioactivity and how does it occur in atomic physics? |  |
| 2. What is the difference between heat and temperature in the context of physics? |  |
| 3. What are latent heat of fusion and vaporization, and why are they important? |  |
| 4. How is the latent heat of fusion and vaporization represented in the NCERT curriculum? |  |
| 5. Can you explain the concept of thermal equilibrium in relation to heat transfer? |  |





















