Class 10 Science: CBSE Sample Question Paper (2020-21) - 1 | Science Class 10 PDF Download
Class-X
Science Theory
TIME: 3 Hrs.
M.M: 80
General Instructions:
Read the following instructions very carefully and strictly follow them:
1. The question paper comprises four sections A, B, C and D. There are 36 questions in the question paper.
All questions are compulsory.
2. Section-A - Question no. 1 to 20 - all questions and parts thereof are of one mark each. These questions contain multiple-choice questions (MCQs), very short answer questions and assertion - reason type questions. Answers to these should be given in one word or one sentence.
3. Section-B - Question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
4. Section-C - Question no. 27 to 33 are short answer type questions, carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
5. Section-D - Question no. - 34 to 36 are long answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
6. There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
7. Wherever necessary, neat and properly labelled diagrams should be drawn.
Section A
Q.1: Give a reason why do chips manufacturers usually flush bags of chips with a gas such as nitrogen? (1 Mark)
Ans: To prevent the oil and fats of the chips from being oxidized or become rancid.
Q.2: State two physical properties of gold which are of extreme use to jewellers. (1 Mark)
Ans: Malleability, ductility, lustrous.
Q.3: Name a cyclic unsaturated carbon compound. (1 Mark)
Ans: Benzene.
Q.4: List any two observations when Ferrous Sulphate is heated in a dry test tube? (1 Mark)
Ans:
- Initial light green colour changes to reddish-brown colour.
- Colourless gas has evolved.
- Gas with a choking smell is evolved.
OR
Identify the products formed when 1 mL of dil. Hydrochloric acid is added to 1g of Sodium metal?
Ans: Sodium chloride and Hydrogen gas
Q.5: Which of the following is not observed in a homologous series? Give the reason for your choice. (1 Mark)
(a) Change in chemical properties
(b) Difference in -CH2 and 14u molecular mass
(c) Gradation in physical properties
(d) Same functional group
Ans: (a)
Solution: Change in chemical properties It does not occur due to the presence of the same functional group.
Q.6: In the arrangement shown in figure, there are two coils wound on a non-conducting cylindrical rod. Initially, the key is not inserted into the circuit. Later the key is inserted and then removed shortly after. (1 Mark)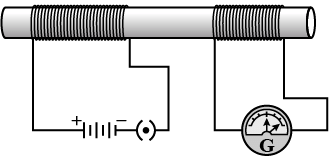
What are the two observations that can be noted from the galvanometer reading?
Ans: There are momentary galvanometer deflections that die out shortly; the deflections are in opposite directions.
Q.7: Draw the magnetic field lines around a straight current-carrying conductor. (1 Mark)
Ans: The field consists of concentric circles centred on the wire.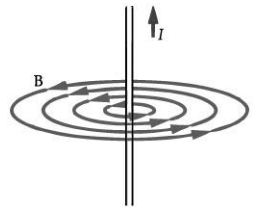
Q.8: Directions: For question number 8, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below? (1 Mark)
Assertion (A): Sodium metal is stored under Kerosene.
Reason (R): Metallic sodium melts when exposed to air.
(a) Both A and R are true and R is the correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true
Ans: (c)
Solution. Sodium is a very reactive metal. It is kept in kerosene to prevent it from coming in contact with oxygen and moisture. If this happens, it will react with the moisture present in the air and form sodium hydroxide. This is a strongly exothermic reaction, and a lot of heat is generated.
Q.9: Directions: For question number 9, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below? (1 Mark)
Assertion (A): Gas bubbles are observed when sodium carbonate is added to dilute hydrochloric acid.
Reason (R): Carbon dioxide is given off in the reaction.
(a) Both A and R are true and R is the correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true
Ans: (a)
Solution: Sodium carbonate reacts with excess hydrochloric acid to form sodium chloride, water and carbon dioxide. In this reaction, bubbles of carbon dioxide are observed.
Q.10: Directions: For question number 10, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below? (1 Mark)
Assertion (A): In the case of a rainbow, the light at the inner surface of the water drop gets internally reflected.
Reason (R): The angle between the refracted ray and normal to the drop surface is greater than the critical angle.
(a) Both A and R are true and R is the correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true.
Ans: (a)
Solution: The rainbow is formed when the light at the inner surface of the water drop gets internally reflected. If the angle between the refracted ray and normal to the drop surface is greater than the critical angle.
Q.11: Directions: Q. No 11 contain five sub-parts each. You are expected to answer any four subparts in this question. (1 Mark)
Read the passage and answer any four questions.
A homologous series is a series of organic compounds which belong to the same family i.e. possess the same functional group) and show similar chemical properties. The members of this series are called homologous and differ from each other by the number of CH2 units in the main carbon chain.
(i) The chemical properties of which of the following compounds is similar to butane?
(a) Butyne
(b) Propene
(c) Propyne
(d) Pentane
Ans: (d)
(ii) The difference between two consecutive members in a homologous series in alkanes in terms of molecular mass and number of atoms of elements are:
(a) 14 a.m.u and CH2 respectively
(b) 12 a.m.u and CH2 respectively
(c) 14 a.m.u and CH3 respectively
(d) 12 a.m.u and CH3 respectively
Ans: (a)
Solution:
Molecular mass = 14 a.m.u. and number of atoms of elements = CH2.
(iii) The compound CH3CH(OH)CH3 belongs to which of the following homologous series?
(a) Aldehydes
(b) Alcohols
(c) Ketones
(d) Carboxylic acids
Ans: (b)
(iv) Which of the following is not the property of a homologous series?
(a) They show similar chemical properties.
(b) They differ by 14 units by mass.
(c) They all contain a double bond
(d) They can be represented by a general formula
Ans: (c)
Solution: They all contain a double bond
(v) Which of the following represent the name and formula of the 2nd member of a homologous series having general formula CnH2n + 2?
(a) Methane, CH4
(b) Ethane, C2H6
(c) Ethene, C2H4
(d) Ethyne (C2H2)
Ans: (a)
Solution: Name — Ethane, Formula — C2H6
Q.12. Why is the refractive index of the atmosphere different at different altitudes? (1 Mark)
Ans. Refractive index of the atmosphere is different at different altitudes because the air density changes with altitudes.
Q.13. What is the magnification of the images formed by the plane mirror and why? (1 Mark)
Ans. Its magnification is +1 because the plane mirror always forms an image equal to the object's size.
Q.14. The electronic configuration of two elements X and Y are 2, 8, 7 and 2, 8, 8, 3 respectively. Write atomic numbers of X and Y ? (1 Mark)
Ans. Atomic number of X = 2, 8, 7 = 17
Atomic number of Y = 2, 8, 8, 3 = 21
Q.15. What is the maximum resistance which can be made using five resistors each of 1/5 W ? (1 Mark)
(a) 1/5 Ω
(b) 10 Ω
(c) 5 Ω
(d) 1 Ω
Ans. (d)
Solution.
The highest resistance is always given by connecting the resistors in series. Here, the highest resistance would be 5 ×1/5 = 1 ohm.
Therefore, the maximum resistance is 1 ohm.
Q.16. The proper representation of the series combination of cells obtaining maximum potential is (1 Mark)
(i)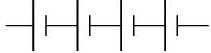
(ii)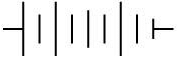
(iii)
(iv) 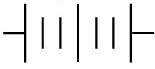
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Ans. (a)
Solution.
Maximum potential is obtained when cells are connected in series such that, the negative terminal of the cell is connected to the positive terminal of the second cell and so on, as shown in the following diagram.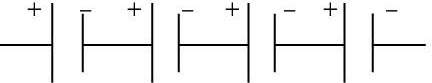
OR
Which of the following represents voltage?
(a) 
(b) Work done × Charge
(c)
(d)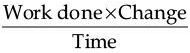
Ans. (a)
Solution. As we know that, Work done = Charge × Potential difference
Since, Charge = Current × time,
Therefore, Work done = (Current × Time) × Potential difference
Or
Potential difference
Q.17. The change in magnetic field lines in a coil is the cause of induced electric current in it. Name the underlying phenomenon. (1 Mark)
Ans. Electromagnetic induction.
Q.18. Answer question numbers 18(a) to 18(d) on the basis of your understanding of the following paragraphs and the related studied concepts.
The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their population. The process of sexual maturation for reproduction is gradual and takes place while general body growth is still going on. Some degree of sexual maturation does not necessarily mean that the mind or body is ready for sexual acts or for having and bringing up children. Various contraceptive devices are being used by human beings to control the size of the population.
(a) List two common signs of sexual maturation in boys and girls. (1 Mark)
Ans. Broadening of shoulder and chest in boys and development of mammary gland or breast in girls.
(b) What is the result of reckless female foeticide? (1 Mark)
Ans. The number of females will become low in comparison to males. Hence, there will be a huge imbalance between the male and female ratio in the population.
(c) Which contraceptive method changes the hormonal balance of the body? (1 Mark)
Ans. Chemical method of contraception e.g. Oral pills.
(d) Write two factors that determine the size of a population. (1 Mark)
Ans. Factors are:
Birth rate and death rate.
Q.19. Veins are thin-walled and have valves. Justify. (1 Mark)
Ans. Veins have thin walls because the blood is no longer under pressure and they have valves to ensure blood flow in one direction.
Q.20. State the role of the pancreas in the digestion of food. (1 Mark)
Ans. The pancreas secretes digestive juice which contains enzymes like trypsin for digesting proteins and lipase for the breakdown of emulsified fats.
Section B
Q.21. Tooth enamel is one of the hardest substances in our body. How does it undergo damage due to eating chocolates and sweets? What should we do to prevent it? (2 Mark)
Ans. When the pH in the mouth is below 5.5, bacteria present in the mouth produce acids by the degradation of sugar and corrode the tooth enamel. It can be prevented by using toothpaste which is generally basic.
Q.22. State the meaning of strong acids and weak acids. Give one example of each. (2 Mark)
Ans. Strong acid: An acid that dissociates completely in water and produces a large number of hydrogen ions. e.g., HCl.
Weak acid: An acid that dissociates partially in water and produces a small amount of hydrogen ions. e.g., CH3COOH.
Q.23. Give the respective scientific terms used for studying :
(i) the mechanism by which variations are created and inherited and,
(ii) the development of a new type of organisms from the existing ones. (2 Mark)
Ans. (i) Genetics is the study of the mechanism by which variations are created and inherited.
(ii) Evolution is used for studying the development of a new type of organisms from the existing ones.
Q.24. On entering in a medium from the air, the speed of light becomes half of its value in air. Find the refractive index of that medium with respect to air. (2 Mark)
Ans. Refractive Index of a medium (µ) 
Let the velocity of light in a vacuum be v1 and velocity of light in the medium be v2.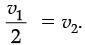
Hence 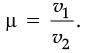
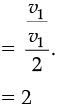
Q.25. List four methods of contraception used by humans? (2 Mark)
Ans. The four methods of contraceptives used by humans are :
(i) Mechanical barrier method.
(ii) Chemical methods
(iii) Oral pills
(iv) Surgical methods
OR
Justify the following statement: “The use of contraceptive methods has a direct effect on the health and prosperity of a family.”
Ans.
- Sexual act always has the potential for pregnancy. Pregnancy makes major demand on the body and mind of a woman and if she is not ready, her health will be adversely affected.
- Contraceptive methods help in avoiding pregnancy and also help in keeping gaps between two children so that women’s bodies recover. These methods help in a limited number of children to one or two.
Q.26. “The number of trophic levels in a food chain is limited.” Give a reason to justify this statement. (2 Mark)
Ans.
- In a food chain, the number of trophic levels is limited to 4-5. This is because according to the 10% law of energy transfer, only 10% of energy passes from one trophic level to the next.
- Thus the amount of energy goes on decreasing with successive trophic levels. After five trophic levels, the existence of organisms would become impossible with such a negligible amount of energy.
OR
We do not clean natural ponds or lakes but an aquarium needs to be cleaned regularly. Why is it so?
Ans. We need to clean the aquarium because of the :
(i) absence of natural decomposer.
(ii) stagnancy of water.
Section C
Q.27. Identify the acid and the base from which sodium chloride is obtained. Which type of salt is it? When is it called rock salt? How is rock salt formed? (3 Mark)
Ans. Acid – Hydrochloric acid/HCl
Base – Sodium hydroxide/NaOH
Neutral Salt
When it forms brown crystals combined with impurities.
Drying up of seas.
OR
(a) For the preparation of cakes, baking powder is used. If at home your mother uses baking soda instead of baking powder, how will it affect the taste of the cake and why?
(b) How is baking soda converted into baking powder?
(c) What makes the cake soft and spongy?
Ans.
(a) The cake will have a bitter taste because of the formation of Na2CO3/sodium carbonate while baking/heating.
(b) By adding tartaric acid
(c) The liberated CO2 gas.
Q.28. (i) Name the element with atomic number 17? (3 Mark)
(ii) To which period does it belong?
(iii)To which group does it belong?
(iv) Write its electronic configuration.
Ans. (i) Chlorine
(ii) 3rd period
(iii) 17th group
(iv) 2, 8, 7.
Q.29. Three resistors of 5 Ω, 10 Ω and 15 Ω are connected in series and the combination is connected to the battery of 30 V. Ammeter and voltmeter are connected in the circuit. Draw a circuit diagram to connect all the devices in the proper correct order. What are the current flowing and potential difference across 10 Ω resistance? (3 Mark)
Ans.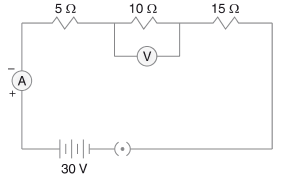
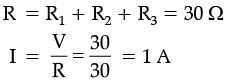
V across 10 Ω = IR = 1 × 10 = 10 V
I across 10 Ω = 1 A
Q.30. (a) Budding, fragmentation and regeneration, all are considered asexual mode of reproduction. Why? (3 Mark)
(b) With the help of neat diagrams, explain the process of regeneration in Planaria?
Ans. (a) Because these methods involve only one parent/organisms are formed as a result of mitotic division/progeny (organisms) are similar in their genetic makeup with no variations.
(b) Planaria can be cut into any number of pieces and each piece grows through specialized cells into a complete organism.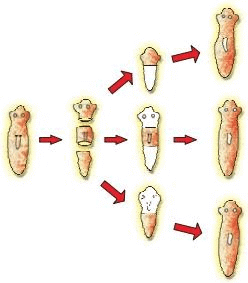
OR
Mention the total number of chromosomes along with the sex chromosomes that are present in a human female and a human male? Explain how in sexually producing organisms the number of chromosomes in the progeny remains the same as that of the parents?
Ans. Human male – 22 pairs of chromosomes along with XY sex chromosome.
Human female – 22 pairs of chromosomes along with XX sex chromosomes.
The original number of chromosomes (the amount of DNA) becomes half during gamete formation. When the gametes fuse, the original number of chromosomes is restored in the progeny.
Detailed Answer
- The total number of chromosomes is 46. In the human male, two sex chromosomes i.e., X or Y are present, while in the human female, both sex chromosomes are X.
- During sexual reproduction, a female gamete or egg cell fuses with a male gamete or sperm cell which are haploid to form a zygote.
- A zygote is a diploid (2n) that contains 46 chromosomes, 23 chromosomes from the mother and 23 from the father. In this way, an equal genetic contribution of male and female parents is ensured in the progeny.
Q.31. In a pea plant, the trait of flowers bearing purple colour (PP) is dominant over white colour (pp). Explain the inheritance pattern of F1 and F2 generations with the help of a cross following the rules of inheritance of traits. State the visible characters of F1 and F2 progenies? (3 Mark)
Ans. Let purple trait be represented by PP and White trait be: pp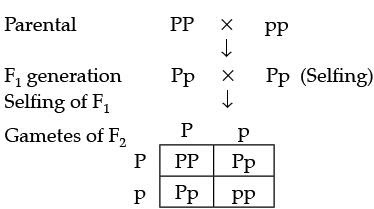
Visible characters of F1 progeny are all flowers are purple coloured and in F2 progenies 3 are purple coloured and 1 is a white coloured flower.
Q.32. The resistance of a wire of 0.01 cm radius is 10 Ω. If the resistivity of the material of the wire is 50 × 10–8W.m, find the length of the wire. (3 Mark)
Ans. Given, Radius, r = 0.01 cm = 0.01 × 10–2 m.
Resistivity, r = 50 × 10–8 Wm.
Resistivity, R = 10 W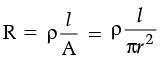
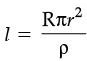
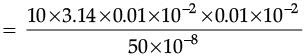
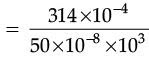
Length = 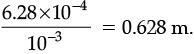
Q.33. What is a solenoid? Draw the field lines of the magnetic field produced on passing a current through and around a current-carrying solenoid. (3 Mark)
Ans. Definition: A coil of many circular turns of insulated copper wire wrapped closely in the shape of a cylinder is called a solenoid.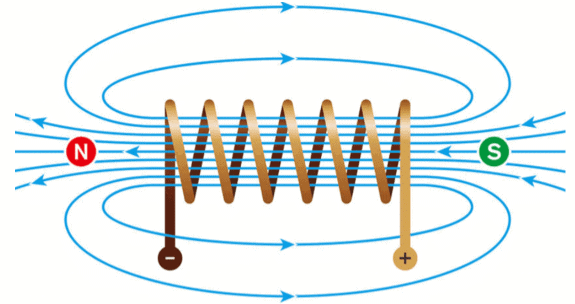 Magnetic field lines through and around a current-carrying solenoid.
Magnetic field lines through and around a current-carrying solenoid.
Section D
Q.34. Carbon cannot reduce the oxides of sodium, magnesium and aluminium to their respective metals. Why? Where are these metals placed in the reactivity series? How are these metals obtained from their ores? Take an example to explain the process of extraction along with chemical equations. (5 Mark)
Ans.
- Metals which are placed high in the reactivity series such as sodium, calcium, magnesium, aluminium etc. are very reactive.
- These metals have a high affinity for oxygen than carbon. Therefore these metals cannot be obtained by reduction with carbon.
- For such metals, the electrolytic reduction process is used for obtaining metal.
Electrolytic reduction of aluminium: Molten aluminium oxide is electrolysed to produce pure aluminium at the cathode while oxygen gas is produced at the anode.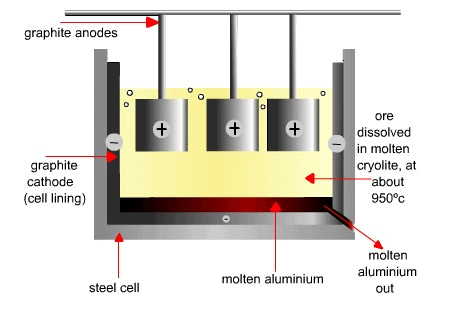 Extraction of AluminiumAl3+ + 3e- → Al (aluminum metal at the (-)cathode)
Extraction of AluminiumAl3+ + 3e- → Al (aluminum metal at the (-)cathode)
2O2- - 4e- → O2 (oxygen gas at the (+)anode)
Q.35. The position of certain elements in the Modern Periodic Table is shown below.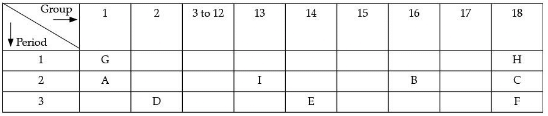
Using the above table answer the following questions giving reasons in each case: (5 Mark)
(i) Which element will form only covalent compounds?
(ii) Which element is a non-metal with valency 2?
(iii) Which element is a metal with valency 2?
(iv) Out of H, C and F which has the largest atomic size.
(v) To which family does H, C and F belong.
Ans. (i) Element E is Silicon. It will form a covalent bond only as it has four electrons in its outermost orbit and needs only four more electrons to become stable.
(ii) Non-metal with valency 2 is B, which is oxygen.
(iii) Element D is a metal with valency 2. Element D is Magnesium. Due to its low electronegativity, it has a higher tendency to donate electrons.
(iv) Element F has the largest atomic size. Element F is argon. Argon occupies 3 energy shells compared to elements H and C, which occupies one and two energy shells. Due to this, the atomic radius of argon is the largest.
(v) Elements H, C and F belong to Group number 18, which means according to their electronic configuration, their octet is complete and thus these elements are stable. They have a very low tendency to react with other elements. Group 18 elements belong to the noble gas family.
OR
Define atomic size. Give its unit of measurement. In the modern periodic table what trend is observed in the atomic radius in a group and a period and why is it so?
Ans. The distance from the centre of the nucleus to the outermost shell of an atom is the atomic radius.
Atomic size is measured in Angstroms, where 1 Angstrom = 10-10 metres. Along the period from left to right: Atomic radius decreases
Reason: Nuclear charge increases which tend to pull the electrons closer to the nucleus. Down the group: Atomic radius increases
Reason: The number of shells increases on going down the group.
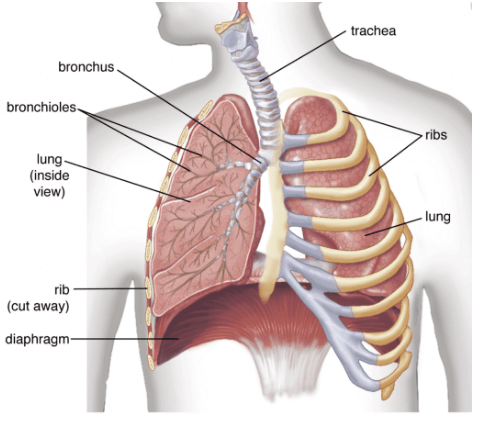
Q.36. (a) Why is there a difference in the rate of breathing between aquatic organisms and terrestrial organisms? Explain.
(b) Draw a diagram of the human respiratory system and label - pharynx, trachea, lungs, diaphragm and alveolar sac on it. (5 Mark)
Ans. (a) Terrestrial organisms can obtain oxygen directly from the air and have a slow breathing rate but aquatic organisms have to obtain oxygen for respiration which is dissolved in water. Since the amount of oxygen dissolved in water is fairly low as compared to the amount of oxygen in the air; the rate of breathing in aquatic organisms is much faster.
(b) Diagram of the human respiratory system :
OR
(a) Name the organs that form the excretory system in human beings.
(b) Describe in brief how urine is produced in the human body.
Ans. (a) Human excretory system comprises a pair of kidneys, a pair of ureters, a urinary bladder and a urethra.
(b) Urine formation involves three steps :
(i) Glomerular filtration: Nitrogenous wastes, glucose, water, amino acids filter from the blood into Bowman’s capsule of the nephron.
(ii) Tubular reabsorption: Useful substances from the filtrate are reabsorbed back by capillaries surrounding the nephron.
(iii) Secretion: Urea, extra water and salts are secreted in the tubule which opens up into the collecting duct and then into the ureter.
|
80 videos|569 docs|80 tests
|
FAQs on Class 10 Science: CBSE Sample Question Paper (2020-21) - 1 - Science Class 10
| 1. What is the duration of the Class X Science Theory exam? |  |
| 2. How many marks is the Class X Science Theory exam worth? |  |
| 3. What is the format of the Class X Science Theory exam? |  |
| 4. Is the Class X Science Theory exam conducted by CBSE? |  |
| 5. Are sample question papers available for the Class X Science Theory exam? |  |






















