Thermal effect
- The rate at which work is done by the source of emf in maintaining the electric current in a circuit is called electric power of the circuit.
P = VI
where V is the potential difference across the conductor, I is current flowing through the conductor. - SI unit of electric power is watt.
1 W = 1 V x l A - Bigger unit of electric power
1 kilowatt (kW) = 103 W
1 megawatt (MW) = 106 W - Other expressions for power
P = I2 R ⇒ P= V2/R - The total workdone (or energy supplied) by the source of emf in maintaining the electric current in the circuit for a given time is called electric energy consumed in the circuit.
- Electric energy = electric power x time
- SI unit of electric energy is joule but another unit is watt hour.
- The bigger unit of electric energy is kilowatt hour (kWh). It is known as Board of Trade Unit (BOT).
- 1 kilowatt hour = 1000 watt x 1 hour
= (1000 J/s) x (3600 s) = 3.6 x 106 J - 1 horse power = 746 watt.
- The electric energy consumed in kWh is given by
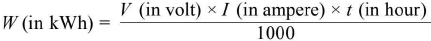
- Whenever the electric current is passed through a conductor, it becomes hot after some time. This indicates that the electric energy is being converted into heat energy. This effect is known as heating effect of current or joule heating effect.
H ∝ I2Rt - H= W = I2Rt joule =
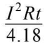 calorie
calorie - Maximum power theorem : It states that the output power of a source of emf is maximum, when external resistance in the circuit is equal to the internal resistance of the source.
- Efficiency of an electric device is defined as the ratio of its output power to the input power.
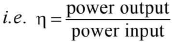
- If 100 W-220 V is written on some electric instruments, then it simply means that on using the instrument on 200 V supply, 100 J of energy is consumed by it in one second.
- When bulbs are joined in parallel, voltage across each bulb will be same. Now, because resistance of highest wattage bulb is minimum, hence heat produced (= V2t/R) (or brightness) will be maximum in highest wattage bulb.
- When bulbs are joined in series, current through each bulb will be same. Now because resistance of lowest wattage bulb is maximum, hence heat produced (= PRt) will be maximum in lowest wattage bulb or lowest wattage bulbs glows with maximum brightness.
- A fuse wire is generally prepared from tin-lead alloy (63% tin + 37% lead). It should have high resistance and low melting point. It is used in series with the electrical installations and protects them from the strong currents.
- Electric iron, electric heater and heating rod are some ofthe important household electrical appliances whose working is based on the heating effect of electric current. In such appliances, the heating element used is of nichrome (an alloy of Ni and Cr).
Chemical effect
- The process of decomposition of electrolyte solution into ions on passing the current through it is called electrolysis. The liquid in which electrolysis take place is called electrolyte.
- Electrodes are two metal plates which are partially dipped in the electrolyte for passing the current through the electrolyte.
- The plate at which current enters the electrolyte is called anode and that at which current leaves the electrolyte is called cathode.
- The vessel in which the electrolysis is carried out is called a voltameter. It contain two electrodes and a solution electrolyte. It is also known as electrolytic cell.
- The ions which carry negative charge and move towards the anode during electrolysis are called anions. The ions formed when chemical reaction involves addition of electrons (i.e. reduction) are called anions.
- The ions which carry positive charge and move towards the cathode during electrolysis are called cations. The ions formed when chemical reaction involves removal of electrons (i.e. oxidation) are called cations.
- Back emf is the emf set up in an electrolytic cell or water voltameter which opposes the external d.c. supply applied to the cell or voltameter.
- The detailed experimental study ofthe electrolysis was carried out by Michael Faraday.
- Faraday’s first law : It states that the mass of substance deposited at the cathode during electrolysis is directly proportional to the quantity of electricity (total charge) passed through the electrolyte.
- m = ZIt, where m is the mass of the substance liberated, Z is the electrochemical equivalent (ECE) of the substance, I is the current passed through the electrolyte. This gives m = Zq.
- SI unit of electrochemical equivalent of a substance is kilogram coulomb-1 (kgC-1). In practice electrochemical equivalent of substance is expressed as gram coulomb-1 (gC-1).
- Faraday’s second law : If some quantity of electricity is passed through different electrolytes, masses of the substance deposited at the respective cathode are directly proportional to their chemical equivalents. m ∝ E , where m is mass o f the ions o f a substance liberated and E is chemical equivalent.
- Chemical equivalent =

- Let m1, m2 is masses of the substance liberated or deposited on various electrode when same current is passed for the same time through their electrolytes and E1,E2 is chemical equivalents of the substance liberated or deposited. The according to Faraday’s second law of electrolysis,
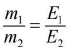
- Faraday’s constant is equal to the ratio of chemical equivalent of a substance to its electrochemical equivalent.
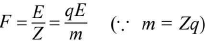
- One faraday = 96500 C/gram equivalent
- 96500 C of charge is required to liberate one gram equivalent of a substance of an electrode during electrolysis.
- Also Faraday’s constant F= Ne, where A is the Avogadro’s number and e is the electronic charge.
- Electroplating is a process of depositing a thin layer of one metal over another metal by the method of electrolysis. The article of cheap metal are coated with precious metal like silver and gold to make them look attractive.
- Anodising is the process of coating aluminium with its oxide electrochemically to protect it against corrosion.
- Electrolysis is used for local anaesthesia (when current is passed through a nerve, it is rendered insensitive to pain), and also for nerve stimulation especially of polio patients.
- An electrochemical cell or simply a cell is an arrangement, in which chemical reaction takes place at a steady rate, so as to convert chemical energy into electrical energy.
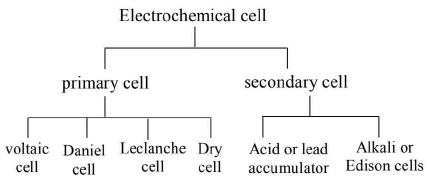
- Primary cell is an electrochemical cell, which once discharge, cannot be put to use again by passing electric current from an external source.
- Secondary cell is an electrochemical cell, which has to be charged initially by passing electric current from an external source, so as to convert the stored chemical energy into electrical energy.
- The initial cost of a primary cell is relatively low as compared to secondary cell, but its operating cost is quite high. The running cost of secondary cells is very low in the long run.
- The capacity of a source of emf is called one ampere hour, if it can discharge one ampere of current for one hour.
- The button cell are usually pellet type, flat in construction and look like a button shape. Owing to their small size, they are used in small electronic devices so as to minimise space and weight.
- The mercury button cell is an alkaline cell of emf 1.36 and zinc-air button cell provides an emf of 1.4 V.
- Chemical effect:
1. For electrolysis of acidulated water practically required minimum voltage is 1.7 volt.
2. For electrolysis D.C. is required.
3. Electrolytic current does not obey ohm’s law except for a very small value of electrolysis.
Thermoelectric effect
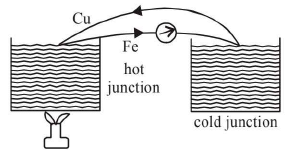
- A thermocouple is an arrangement of two suitable dissimilar metallic wires (wires of different materials) joined at their ends to form junctions.
- The conversion of heat into electrical energy by using a thermocouple is called thermoelectricity.
- Seebeck effect is the phenomenon of generation of an electric current in a thermocouple by keeping its two junctions at different temperatures
.
- Applications
(i) For the measurement of current (AC and DC) apparatus.
(ii) For the measurement of temperature - Direction of thermo-current
Hot Coffee ⇒ thermo electric current from Cu to Fe through hot junction.ABC ⇒ Current from Antimony (Sb) to Bismuth via Cold junction.
The thermo emf developed in a thermocouple depends upon the nature of metals forming the thermo-couple and difference in temperature of the two junctions.
On the basis of Seebeck experimental results, he arranged a number of metals in the form of a series, called thermoelectric series.
The series is given below.
Bi, Ni, Co, Pd, Pt, Cu, Mn, Hg, Pb, Sn, Au, Zn, Cd, Fe, Sb, Te.Seebeck also found experimentally that for a given difference of temperatures of two junctions, the larger is the gap in Seebeck series between the metals joining the thermocouple, the greatest will be the thermo emf generated.
The temperature of hot junction at which thermo emf in a thermocouple is maximum is called neutral temperature.
The value of neutral temperature is a constant for thermocouple. Its value depends upon the nature of material forming the thermocouple and independent of the temperature of the cold junction.
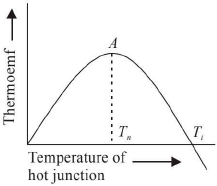
The temperature of hot junction at which the thermo- emf in a thermocouple become zero and just beyond, it reverses its direction is called temperature of inversion.
The value of temperature of inversion depends upon the temperature of the cold junction and the nature of materials forming the thermocouple.
Relation between thermo emf and temperature of hot junction.
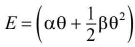
where α and β are constants called thermo electric constants.The rate of change of thermo emf with change in the temperature of the hot junction is called thermoelectric power.
A thermopile is a combination of a large number of thermocouples in series. It can be used to detect the heat radiations.
Peltier effect : If a current is passed round the conductors of a thermo couple, one junction gets heated and other junction gets cooled. The phenomenon is called Peltier effect. It is a reversible effect. The amount of heat absorbed or evolved depends on charge passed.
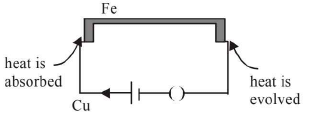
Heat: H (joule) ∝ Q coulomb
H = πQ = πIt; π = Peltier coefficient or Peltier emfThomson effect : If a current is sent through a single unequally heated conductor absorption or evolution of heat takes place throughout the conductor or if a temperature difference exists between two points of a conductor, a potential difference is developed between the points. This phenomenon is called Thomson effect. It is also reversible effect. For lead (Pb) Thomson coefficient is zero.
Thermoelectric thermometer : It is a device used to measure both low and high temperatures. Thermoelectric thermometers have much wider range of measurement of temperature (from -200°C to 1600°C). They are quite sensitive and can measure temperature accurately upto 0.05°C. Disadvantage of thermometer is that it does not give direct reading and hence it cannot be used in experiments on calorimetry.


















