Particle Nature of Light, Wave Nature of Matter & de Broglie's Equation | Physics for JEE Main & Advanced PDF Download
Particle Nature of Light
The emission of free electrons from a metal surface when the light is shone on it, it is called the photoemission or the photoelectric effect. This effect led to the conclusion that light is made up of packets or quantum of energy. Now the question was whether the light quantum theory was indicative of the particle nature of light. Einstein already associated the light quantum with momentum. This strongly supported the particle nature of light and these particles were named photons. Thus, the wave-particle duality of light came into the picture.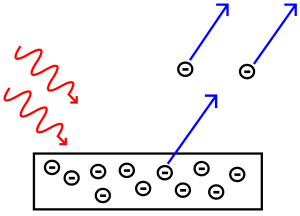 Photoelectric Emission
Photoelectric Emission
Photons
Some points to be kept in mind are:
- A photon is an elementary particle. It is a quantum of light.
- Energy of a photon is given by E = hν. Its momentum is p = hν / c and speed is c, which is the speed of light.
- Irrespective of the intensity of radiation, every photon of a frequency v has the same momentum p = h / νc and energy E = hν.
- The increase in the intensity of light only increases the number of photons crossing an area per unit time. It does not affect the energy of the radiation.
- A photon remains unaffected by electric and magnetic fields. It is electrically neutral.
- A photon has a zero mass, i.e. it is massless.
- It is a stable particle.
- Photons can be created or destroyed when radiation is emitted or absorbed.
- The total energy and momentum are conserved during a photon-electron collision.
- A photon cannot decay on its own.
- The energy of a photon can be transferred during an interaction with other particles.
- A photon is a spin-1 particle, unlike electrons which are ½ spin. It’s spin axis is parallel to the direction of travel. It is this property of photons which supports the polarization of light.
Wave Nature of Matter
The wave nature of matter is one of the most counter-intuitive concepts in Physics. You have seen examples of both particle nature of light and wave nature of light. You know about the Photoelectric effect due to Albert Einstein’s courtesy.
In the photoelectric effect, the electrons and photons exhibit the properties of a particle, just like a billiard ball. But you surely remember the Diffraction experiment and the Interference Rings. Just like how two ripples on the surface of a pond interact. We see the wave nature of light in these cases. It’s an amazing mystery. It even involves our sight! The gathering and focusing mechanism of light by the eye-lens conform to the wave nature of light. But its absorption by the rods and cones of the retina conforms to the particle nature of light! While we were still struggling to understand this mystery, along came Louis de Broglie to make it even more complicated with his de Broglie Relation.
De Broglie’s Equation
De Broglie’s hypothesis stated that there is symmetry in nature and that if light and radiation behave as both particles and waves, matter too will have both the particle and wave nature.
λ = h / p = h / mv
Through the de Broglie’s relationship, we now had a wave theory of matter. The ‘Lambda’ here represents the wavelength of the particle and ‘p’ represents the momentum of the particle. The significance of the de Broglie relationship is that it proves mathematically that matter can behave as a wave. In layman terms, de Broglie equation says that every moving particle – microscopic or macroscopic –has its own wavelength.
For macroscopic objects, the wave nature of matter is observable.
For larger objects, the wavelength gets smaller with the increasing size of the object, quickly becoming so small as to become unnoticeable which is why macroscopic objects in real life don’t show wave-like properties. Even the cricket ball you throw has a wavelength that is too small for you to observe. The wavelength and the momentum in the equation are connected by the Plank’s constant.
Heisenberg’s Uncertainty
The Davisson-Germer experiment proved beyond doubt the wave nature of matter by diffracting electrons through a crystal. In 1929, de Broglie was awarded the Nobel Prize for his matter wave theory and for opening up a whole new field of Quantum Physics. The matter-wave theory was gracefully incorporated by Heisenberg’s Uncertainty Principle. The Uncertainty Principle states that for an electron or any other particle, both the momentum and position cannot be known accurately at the same time. There is always some uncertainty with either the position ‘delta x’ or with the momentum, ‘delta p’.
Heisenberg’s Uncertainty Equation:
σxσp ≤ h / 2
Say you measure the momentum of the particle accurately so that ‘delta p’ is zero. To satisfy the equation above, the uncertainty in the position of the particle, ‘delta x’ has to be infinite. From de Broglie’s equation, we know that a particle with a definite momentum has a definite wavelength ‘Lambda’. A definite wavelength extends all over space all the way to infinity. By Born’s Probability Interpretation this means that the particle is not localized in space and therefore the uncertainty of position becomes infinite.
In real life though, the wavelengths have a finite boundary and are not infinite and thus both the position and momentum uncertainties have a finite value. De Broglie’s equation and Heisenberg’s Uncertainty Principle are apples of the same tree.
|
268 videos|740 docs|171 tests
|





















