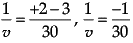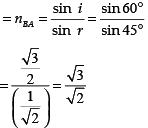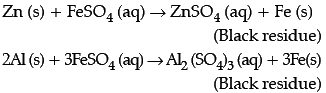Class 10 Science: CBSE Sample Question Paper- Term I (2021-22) - 4 | Science Class 10 PDF Download
| Table of contents |

|
| Class-XTime: 90 MinutesM.M: 40 |

|
| Section - A |

|
| Section - B |

|
| Section - C |

|
Class-X
Time: 90 Minutes
M.M: 40
General Instructions:
Read the following instructions very carefully and strictly follow them:
- The Question Paper contains three sections.
- Section A has 24 questions. Attempt any 20 questions.
- Section B has 24 questions. Attempt any 20 questions.
- Section C has 12 questions. Attempt any 10 questions.
- All questions carry equal marks.
- There is no negative marking.
Section - A
Q.1: Plaster of Paris is
(a) (CaSO4). ½ H2O
(b) CaSO4.2H2O
(c) CaSO4.H2O
(d) CaSO4
Correct Answer is Option (a)
Plaster of Paris is Calcium Sulphate Hemihydrate - CaSO4. ½H2O.
Q.2: Which of the following statements is correct about water of crystallization?
(a) Crystals of salts obtain their shape.
(b) Crystals of salts obtain their colour.
(c) Crystals of salts form a part of crystal structure.
(d) All of the above
Correct Answer is Option (d)
Water of crystallization forms a part of crystal structure. They also obtain their shape and colour.
Q.3: The gas which is passed through dry slaked lime to produce bleaching powder is:
(a) H2
(b) O2
(c) Cl2
(d) N2
Correct Answer is Option (c)
Bleaching powder is produced by the action of chlorine on dry slaked lime Ca(OH)2. Chlorine is produced during the electrolysis of aqueous sodium chloride.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Q.4: A milk man adds a very small amount of baking soda to fresh milk:
(a) To increase the rate of fermentation
(b) To decrease the rate of fermentation
(c) To increase its quality
(d) To make paneer
Correct Answer is Option (b)
A milk man adds a very small amount of baking soda to fresh milk to decrease the rate of fermentation. The milkman shifts the pH of the fresh milk from six to slightly alkaline because in alkaline condition, milk does not set as curd easily.
Q.5: The compound which dissolves in water to give a solution with a pH greater than 7 is:
(a) Calcium Carbonate
(b) Copper (II) Hydroxide
(c) Sodium Hydroxide
(d) Sulphur Dioxide
Correct Answer is Option (c)
Sodium hydroxide is an alkali that dissolves in water to form a solution of pH as greater than 7.
Q.6: Which of these is an amphoteric oxide?
(a) Al2O3
(b) P2O5
(c) N2O
(d) Fe2O3
Correct Answer is Option (a)
Metal oxides which react with both acids as well as bases to produce salt and water are known as amphoteric oxides. Example includes aluminium oxide, zinc oxide, etc.
Q.7: Which pair of compounds is not ionic?
(A) KCl and HCl
(B) CCl4 and CH4
(C) CCl4 and NaCl
(D) KCl and CCl4
Correct Answer is Option (b)
CCl4 and CH4 are not ionic compounds. They are neutral molecule and doesn’t form positive or negative ion.
Q.8: The element that liberate H2 gas on reacting with diluted nitric acid is:
(a) Zn and Mg
(b) Mn and Sn
(c) Mg and Mn
(d) Mn and Fe
Correct Answer is Option (c)
Dilute nitric acid can react only with Mg and Mn that releases hydrogen gas.
Q.9: Shipra observed the rate of evolution of hydrogen gas with five metals P, Q, R, S and T at room temperature. 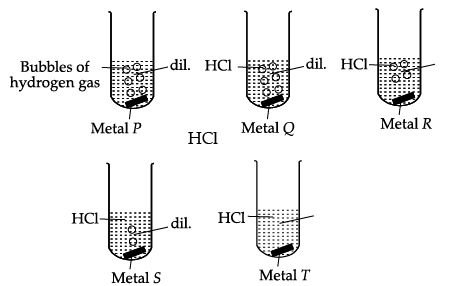
Based on above observation, the metals P, Q, R, S and T are: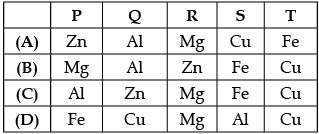
(a) A
(b) B
(c) C
(d) D
Correct Answer is Option (b)
In reactivity series, the order of reactivity of given metals is as follows: Mg > Al > Zn > Fe > Cu Thus, rate of evolution of H2 gas in case of Mg is maximum followed by Al, Zn and then Fe. Cu does not react with dil. HCl, So there is no evolving of H2 gas is observed.
Q.10: ‘Although nitrogen is the most abundant gas in the atmosphere, it does not combustion’. Identify the correct reason for this statement.
(a) Nitrogen is a reactive gas
(b) Nitrogen is an inert gas
(c) Nitrogen is an explosive gas
(d) Only hydrocarbons can take part in combustion
Correct Answer is Option (b)
The triple bond in nitrogen is too strong to be broken and hence it is an inert gas which does not take part in combustion.
Q.11: Which of the following statements about the autotrophs is incorrect?
(a) They synthesise carbohydrates from carbon dioxide and water in the presence of sunlight and chlorophyll.
(b) They store carbohydrates in the form of starch.
(c) They convert carbon dioxide and water into carbohydrates in the absence of sunlight.
(d) They constitute the first trophic level in food chains
Correct Answer is Option (c)
Autotrophs take in food from the outside world and convert them into stored forms of energy. This material is taken in the form of carbon dioxide and water which is converted into carbohydrates in the presence of sunlight and chlorophyll.
Q.12: Which of the following completes the given equation?
Glucose + Oxygen → (?)
(a) Only carbon dioxide + water + energy
(b) Only carbon dioxide + water
(c) Only carbon dioxide
(d) Only water + energy
Correct Answer is Option (a)
The given equation represents aerobic respiration.
Glucose + oxygen → carbon dioxide + water + energy
Q.13: The inner lining of stomach is protected by one of the following from hydrochloric acid. Choose the correct one.
(a) Pepsin
(b) Mucus
(c) Salivary amylase
(d) Bile
Correct Answer is Option (b)
The stomach has a lining of mucus cells. The mucus is secreted in the gastric juice by the glands present in the stomach wall. It helps to protect the wall of stomach from its own secretions of hydrochloric acid. If mucus is not secreted, HCl will cause the erosion of inner lining of stomach leading to ulcer formation.
Q.14: During deficiency of oxygen in tissues of human beings, pyruvic acid is converted into lactic acid in the
(a) Cytoplasm
(b) Chloroplast
(c) Mitochondria
(d) Golgi body
Correct Answer is Option (a)
Lactic acid is formed after anaerobic respiration in muscle cells and this happens in cytoplasm.
Q.15: What prevents back flow of blood inside the heart during contraction?
(a) Valves in heart
(b) Thick muscular walls of ventricles
(c) Thin walls of atria
(d) All of the above
Correct Answer is Option (a)
Valves ensure that blood does not flow backwards when the atria or ventricles contract. Semilunar valves, the valves present between ventricles and their attached vessels, serve to prevent the backflow of blood to ventricles from their respective attached vessels. Likewise, atrioventricular (AV) valve between atrium and ventricle directs the flow of blood and prevents any backflow into atria.
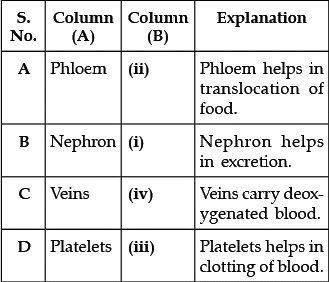
Q.16: Match the words of Column (A) with that of Column (B) 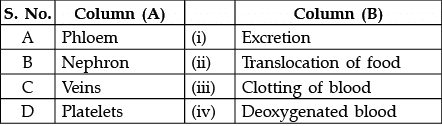
(a) A - (ii), B - (i), C - (iv), D - (iii)
(b) A - (iii), B - (ii), C - (i), D - (iv)
(c) A - (iv), B - (iii), C - (ii), D - (i)
(d) A - (i), B - (iv), C - (iii), D - (iv)
Correct Answer is Option (a)
Q.17: In which of these cases, a real image, equal in size to the object, is obtained?
(a) When an object is placed at the centre of curvature in front of a concave mirror.
(b) When an object is placed at the centre of curvature in front of a plane mirror.
(c) When an object is placed at the centre of curvature in front of a convex mirror
(d) None of these
Correct Answer is Option (a)
A real image, equal in size to the object, is obtained when the object is placed at the centre of curvature in front of a concave mirror.
Q.18: Choose the incorrect statement.
(a) A concave mirror can give a virtual image
(b) A convex mirror can give a virtual image
(c) A convex lens can give a virtual image
(d) All of these
Correct Answer is Option (a)
Concave mirrors can produce both real and virtual images; they can be upright (if virtual) or inverted (if real); they can be behind the mirror (if virtual) or in front of the mirror (if real); they can also be enlarged, reduced, or the same size as object.
Q.19: Upon which of these factors the amount of light reflected depends?
(a) Nature of material of the object
(b) Nature of the surface
(c) Smoothness of the surface
(d) All of these
Correct Answer is Option (d)
The amount of light reflected depend upon the properties of the material and the surface polished. Hence reflection depends upon the smoothness of the material at which light is incident.
Q.20: In order to get a diminished and virtual image, the object can be placed anywhere in front of a
(a) Concave mirror
(b) Plane mirror
(c) Convex mirror
(d) None of these
Correct Answer is Option (c)
Convex mirror always form virtual, erect and diminished image irrespective of object distance.
Q.21: Which of the following correctly represents the incident ray, the refracted ray and the emergent ray in the given glass slab?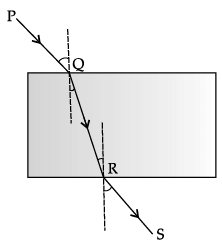
(a) A
(b) B
(c) C
(d) D
Correct Answer is Option (a)
PQ is incident ray, QR is refracted ray and RS is the emergent ray in the given glass slab.
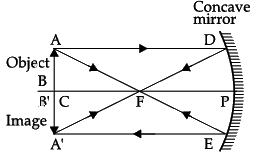
Q.22: When an object of size 1 cm is placed at a distance of 15 cm from a concave mirror of focal length 10 cm, the position, nature and size of the image formed will be
(a) On the left side, 30 cm, real, inverted and magnified
(b) On the left side, 20 cm, virtual, upright and diminished
(c) On the right side, 30 cm, real, inverted and magnified
(d) On the right side, 20 cm, virtual, upright and diminished
Correct Answer is Option (a)
1/v + 1/u = 1/f
We get,
1/v + (1/-15) = 1/(-10)
So, image distance, v = –30 cm on the left side of the mirror. It is real, inverted and magnified.
Q.23: Which of these options is correct in case of concave mirror?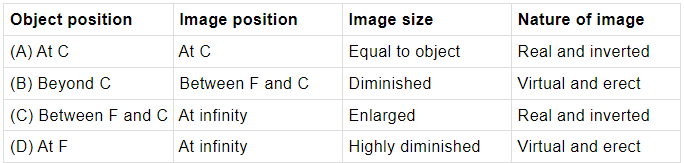
(a) A
(b) B
(c) C
(d) D
Correct Answer is Option (a)
In case of concave mirror, when the object is at C, the image formed is at C, image will be real, inverted and of same size as the object.
Q.24: Choose the correct statement regarding the propagation of light of different colours of white light in air?
(a) Red light moves fastest
(b) Blue light moves faster than green light
(c) All the colours of the white light move with the same speed
(d) Yellow light moves with the mean speed as that of the red and the violet light.
Correct Answer is Option (b)
Blue light moves faster than green light.
Section - B
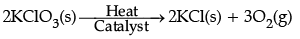
Which of the following statement(s) is (are) correct about the reaction?
(a) It is a decomposition reaction and endothermic in nature.
(b) It is a combination reaction.
(c) It is a decomposition reaction and accompanied by release of heat.
(d) It is a photochemical decomposition reaction and exothermic in nature.
Correct Answer is Option (a)
It is a decomposition reaction and endothermic in nature because decomposition requires heat for products formation.
Q.26: In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Correct Answer is Option (b)
Lead acetate can be used in place of lead nitrate because like lead nitrate, it is also a soluble salt in water. The reaction is as follows :
Pb(CH3COO)2 + 2KI → 2PbI2 + 2CH3COO-K+
Lead sulphate is insoluble in water and will not dissociate into Pb2+ ions, so it cannot be used.
Q.27: A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4.
(b) FeSO4 acts as an oxidising agent and oxidises KMnO4.
(c) The colour disappears due to dilution; no reaction is involved.
(d) KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound.
Correct Answer is Option (a)
A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. A permanganate solution is usually purple in colour. The light purple colour of the solution fades and finally disappears. This is because potassium permanganate (KMnO4) is relatively an unstable compound, it tends to decompose in the presence of ferrous sulphate (FeSO4). This changes the colour of the solution from purple to colourless. FeSO4 gets oxidised to Fe2(SO4) as KMnO4 acts as a good oxidising agent in an acidic medium.
Q.28: A compound is prepared from gypsum upon heating to a temperature of 373 K and it changes back to gypsum on adding water. Which is the incorrect statement about the compound?
(a) The compound is used for setting fractured bones.
(b) The compound is called plaster of Paris which is calcium sulphate dehydrate with a formula CaSO4.2HO.
(c) If heated at higher temperature, the compound becomes dehydrated and is called dead burnt plaster.
(d) Both (a) and (b).
Correct Answer is Option (b)
The compound is called plaster of Paris which is calcium sulphate hemihydrate with a formula CaSO4.1/2H2O.
Q.29: In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus (Figure) was set up. Which among the following statement(s) is (are) correct?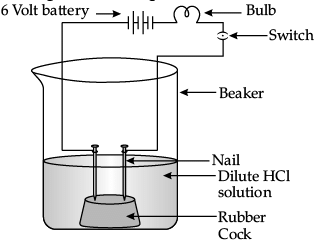 (i) Bulb will not glow because electrolyte is not acidic.
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) Only
Correct Answer is Option (c)
Bulb will glow because NaOH is a strong base and strong acids or bases are good conductors of electricity as they ionise completely in aqueous solution to give ions that furnishes ions for conduction.
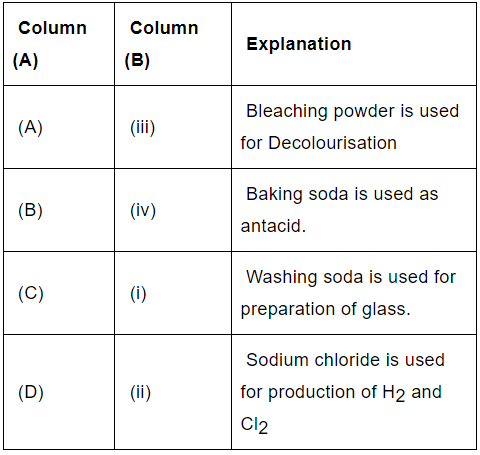
Q.30: Match the chemical substances given in Column (A) with their appropriate application given in Column (B)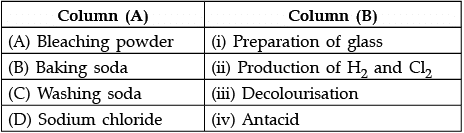
(a) A—(ii), B—(i), C—(iv), D—(iii)
(b) A—(iii), B—(ii), C—(iv), D—(i)
(c) A—(iii), B—(iv), C—(i), D—(ii)
(d) A—(ii), B—(iv), C—(i), D—(iii)
Correct Answer is Option (c)
Q.31: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): In the given equation, ‘X’ stands for 2.
3Fe + XH2O → Fe3O4 + 4H2
Reason (R): To balance the given equation the number of atoms of each element should be same on both the sides.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is NOT the correct explanation of A.
(c) A is true but R is false.
(d) A is false and R is true.
Correct Answer is Option (d)
To balance the given equation the number of atoms of each element should be same on both the sides. Hence, the ‘X’ value should be 3.
3Fe + 4H2O → Fe3O4 + 4H2
Q.32: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): If a few drops of concentrated acid accidentally spills over the hand of a student, he washed the hand immediately with plenty of water and applied the paste of baking soda.
Reason (R): A strong base cannot be used to neutralize the acid due to its corrosive nature.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is NOT the correct explanation of A.
(c) A is true but R is false.
(d) A is false and R is true.
Correct Answer is Option (a)
Washing the hand immediately with plenty of water as it helps to wash away most of the acid. Thereafter, applying a paste of baking soda (NaHCO3) to neutralize the little acid if any, left over the hand. Baking soda has weak basic properties that are used to neutralize the acid. Here, a strong base can be used to neutralize the acid as it can corrode the skin.
Q.33: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): The substance “X” in the given substance is potassium hydroxide, which absorbs CO2.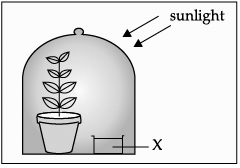 Reason (R): Potassium hydroxide releases carbon dioxide for the process of photosynthesis to occur.
Reason (R): Potassium hydroxide releases carbon dioxide for the process of photosynthesis to occur.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is NOT the correct explanation of A.
(c) A is true but R is false.
(d) A is false and R is true.
Correct Answer is Option (c)
The substance kept in a glass beaker is potassium hydroxide. It absorbs CO2.
Q.34: Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion (A): A mirror having a very wide field of view must be convex.
Reason (R): It forms virtual, erect and diminished image for object placed at infinity.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is NOT the correct explanation of A.
(c) A is true but R is false.
(d) A is false and R is true.
Correct Answer is Option (a)
Convex mirrors have a very wide field of view. Hence, used in vehicles for rear view. It forms virtual, erect and diminished image for object placed at infinity.
Q.35: 2 mL each of concentrated HCl, HNO3 and a mixture of concentrated HCl and concentrated HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be
(a) Al
(b) Au
(c) Cu
(d) Pt
Correct Answer is Option (b)
Aqua regia is a mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3 : 1. It can dissolve gold, even though neither of these acids can do so alone. Aqua regia is a highly corrosive, fuming liquid. It is one of the few reagents that is able to dissolve gold and platinum.
Q.36: Reaction between X and Y, forms compound Z. X loses electron and Y gains electron. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
Correct Answer is Option (b)
Ionic compounds are formed by the transfer of electrons. Ionic compounds conduct electricity in their molten state, occur as solid and have high melting and boiling points. Low melting point is not the character of ionic compounds. Compound Z is formed because of transfer of electrons from X to Y, this means Z is an ionic compound which could not have low melting point.
Q.37: Which of the following options represents normal blood pressure?
(a) 120/80 mm of Hg
(b) 120/60 mm of Hg
(c) 180/80 mm of Hg
(d) 160/90 mm of Hg
Correct Answer is Option (a)
Normal blood pressure is represented by 120/80 mg of Hg.
Q.38: The enzyme which converts starch into sugar present in saliva is :
(a) Pepsin
(b) Hydrochloric Acid
(c) Amylase
(d) Insulin
Correct Answer is Option (c)
Salivary glands secretes an enzyme called salivary amylase or ptyalin. This enzyme digests starch and converts it into sucrose or maltose.
Q.39: The aim of the given experiment is to show: 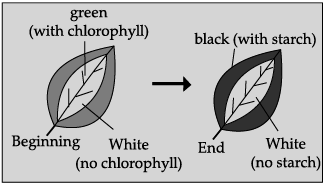 (a) Chlorophyll is necessary for photosynthesis
(a) Chlorophyll is necessary for photosynthesis
(b) Sunlight is necessary for photosynthesis
(c) Oxygen is released as a result of photosynthesis
(d) Carbon dioxide is released during the process.
Correct Answer is Option (a)
The given figure demonstrate that chlorophyll is essential for photosynthesis. Chlorophyll traps carbon dioxide, which is an important raw material for photosynthesis.
Q.40: Identify blood vessels X, Y and Z.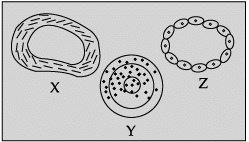

(a) A
(b) B
(c) C
(d) D
Correct Answer is Option (c)
In the given figure X represents vein, Y represents artery and Z, capillary.
Q.41: How Amoeba respires ?
(a) By the process of transpiration
(b) By osmosis
(c) By diffusion
(d) By inhalation
Correct Answer is Option (c)
In Amoeba respiration take place through its body surface by diffusion.
Q.42: A beam of Light is incident through the holes on side A and emerges out of the holes on the other face of the box as shown in the figure. Which of the following could be inside the box?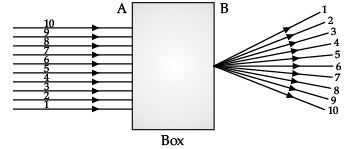
(a) Concave lens
(b) Rectangular glass slab
(c) Prism
(d) Convex lens
Correct Answer is Option (d)
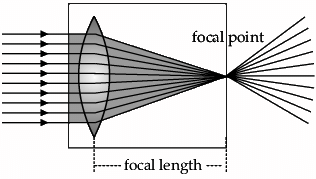
In the given diagram, parallel rays converge at a point and emerges from face B. So, there will be a convex lens inside the box.
Q.43: The path of a ray of light coming from air passing through a rectangular glass slab traced by four students shown as A, B, C, and D in the figure. Which one of them is correct?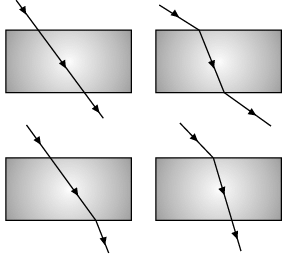 (a) A
(a) A
(b) B
(c) C
(d) D
Correct Answer is Option (b)
A light ray passing through a glass slab obliquely will be emerging parallel to the direction of the incident ray.
Q.44: Figure shows a ray of light as it travels from medium A to medium B. Refractive index of the medium B relative to medium A is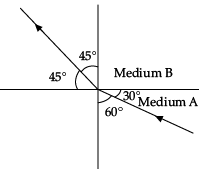
(a) √3/√2
(b) √2/√3
(c) 1/√2
(d) √2
Correct Answer is Option (a)
Here, angle of incidence = i = 60° Angle of refraction = r = 45°
Refractive index of the medium B relative to medium A
Q.45: Beams of light are incident through the holes A and B and emerge out of box through the holes C and D respectively as shown in the figure. Which of the following could be inside the box?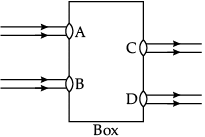
(a) A rectangular glass slab
(b) A convex lens
(c) A concave less
(d) A prism
Correct Answer is Option (a)
In the given figure, emergent light rays are parallel to the direction of incident light rays. Out of the given options only a rectangular glass slab can change the path of light ray in such a way that emergent rays are parallel to the incident rays.
Q.46: Which of the following ray diagrams is correct for the ray of light incident on a concave mirror as shown in figure?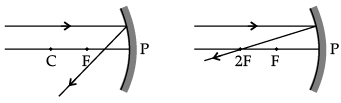
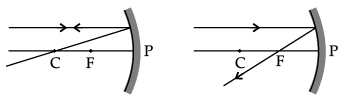 (a) Fig. A
(a) Fig. A
(b) Fig. B
(c) Fig. C
(d) Fig. D
Correct Answer is Option (d)
Ray of light incident parallel to the principal axis towards the mirror after reflection will pass through the principal focus. So, Figure D is correct.
Q.47: A prism ABC (with BC as base) is placed in different orientations. A narrow beam of white light is incident on the prism as shown in Figure. In which of the following cases, after dispersion, the third colour from the top corresponds to the colour of the sky?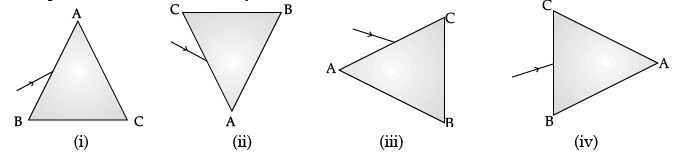 (a) (i)
(a) (i)
(b) (ii)
(c) (iii)
(d) (iv)
Correct Answer is Option (b)
If prism is kept with base BC at bottom, then the emergent band of colour would show violet at the bottom. If prism is kept with base BC at top, then violet would be at top; followed by indigo and blue.
Q.48: A man wearing yellow coloured glass for left eye and red coloured glass for right eye stands in front of a plane mirror. Choose the correct statement about his image in the mirror is correct?
(a) Both the eyes appear red.
(b) Both the eyes appear yellow.
(c) Left eye appears yellow and right eye appears red.
(d) Left eye appears red and right eye appears yellow.
Correct Answer is Option (d)
When a man wearing yellow coloured glass for left eye and red coloured glass for right eye and stands in front of a plane mirror, he observes his image in the mirror as left eye coloured red and right coloured yellow due to lateral inversion.
Section - C
Observe the given experimental set-up carefully and answer any of the four questions.
I. In an experiment, Vaani took 10 mL of freshly prepared iron sulphate solution in four different test tubes.
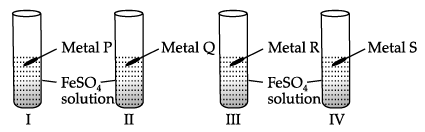 She then added four different metal strips to each test tube as shown below. She observed the following results:
She then added four different metal strips to each test tube as shown below. She observed the following results:A. In test tubes I and III, black residue was obtained.
B. In test tubes II and IV, no change was observed.
Black residue was obtained in test tubes I and III. This shows that
I. P is more reactive than iron.
II. R is more reactive than iron
III. Q is less reactive than iron
IV. S is less reactive than iron
(a) I and II are correct.
(b) III and IV are correct
(c) I and III are correct
(d) II and IV are correct
Correct Answer is Option (a)
Black residue is obtained in test tubes I and III i.e., iron gets displaced in test tubes I and III. Therefore, metals P and R should be more reactive than iron.
Observe the given experimental set-up carefully and answer any of the four questions.
I. In an experiment, Vaani took 10 mL of freshly prepared iron sulphate solution in four different test tubes.
 She then added four different metal strips to each test tube as shown below. She observed the following results:
She then added four different metal strips to each test tube as shown below. She observed the following results:A. In test tubes I and III, black residue was obtained.
B. In test tubes II and IV, no change was observed.
Why no change is observed in test tubes II and IV ?
(a) Metals Q and S should be less reactive than iron.
(b) Metals P and Q are more reactive than iron.
(c) Metals P and S are less reactive than iron
(d) Metals Q and S are more reactive than iron.
Correct Answer is Option (a)
No change is observed in test tubes II and IV hence, metals Q and S should be less reactive than iron.
Observe the given experimental set-up carefully and answer any of the four questions.
I. In an experiment, Vaani took 10 mL of freshly prepared iron sulphate solution in four different test tubes.
 She then added four different metal strips to each test tube as shown below. She observed the following results:
She then added four different metal strips to each test tube as shown below. She observed the following results:A. In test tubes I and III, black residue was obtained.
B. In test tubes II and IV, no change was observed.
Metals P, Q, R and S could be respectively
(a) Al, Cu, Pb, Ag
(b) Pb, Cu, Ag, Al
(c) Zn, Al, Cu, Ag
(d) Zn, Cu, Al, Ag
Correct Answer is Option (d)
Black residue is obtained in test tubes I and III i.e., iron gets displaced in test tubes I and III. Therefore, metals P and R should be more reactive than iron. No change is observed in test tubes II and IV hence, metals Q and S should be less reactive than iron. Hence, Metal P could be zinc (Zn), Q could be copper (Cu), R could be Aluminium (Al) and S could be Silver (Ag).
Observe the given experimental set-up carefully and answer any of the four questions.
I. In an experiment, Vaani took 10 mL of freshly prepared iron sulphate solution in four different test tubes.
 She then added four different metal strips to each test tube as shown below. She observed the following results:
She then added four different metal strips to each test tube as shown below. She observed the following results:A. In test tubes I and III, black residue was obtained.
B. In test tubes II and IV, no change was observed.
What type of reaction is seen in test tubes I and III?
(a) Combination reaction.
(b) Displacement reaction
(c) Redox reaction
(d) Neutralisation reaction
Correct Answer is Option (b)
The black residue is seen in test tube I and III due to the formation of iron. This type of reaction is called displacement reaction.
Observe the given human digestive system carefully and answer any of the four questions.
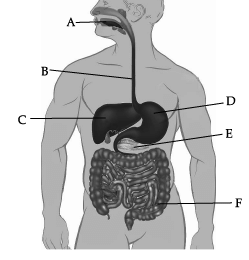 Which of these correctly represent the labels B, C, D and E?
Which of these correctly represent the labels B, C, D and E? (a) B- Oesophagus, C- Liver, D- Stomach, E- pancreas
(b) B- Pancreas, C- Oesophagus, D- Liver, E- Stomach
(c) B- Stomach, C- Pancreas, D- Oesophagus, E- Liver
(d) B- Liver, C- Stomach, D- Pancreas, E- Oesophagus
Correct Answer is Option (a)
In the given picture of human digestive system, B is Oesophagus, C is Liver, D is Stomach, and E is pancreas.
Observe the given human digestive system carefully and answer any of the four questions.
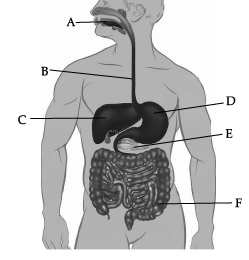
(a) Bile
(b) Pepsin
(c) Saliva
(d) Gastric juice
Correct Answer is Option (a)
Label C represents liver. Liver secretes bile, which is stored in gall bladder.
Observe the given human digestive system carefully and answer any of the four questions.
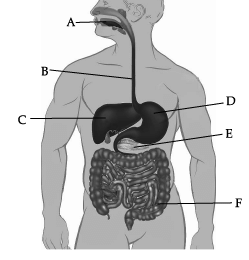
(a) Bile juice
(b) Pancreatic juice
(c) Ptyalin
(d) Pepsin
Correct Answer is Option (a)
Bile juice doesn’t contain any enzyme. It helps in digestion of fats.
Observe the given human digestive system carefully and answer any of the four questions.

(a) Absorption of food
(b) Absorption of water
(c) Secretion of hormones
(d) Removal of waste material
Correct Answer is Option (b)
Absorption of water is not occurring normally in region F (Large intestine).
A narrow beam of white light is passing through a glass prism as shown in the diagram. Study the diagram and answer the following questions.
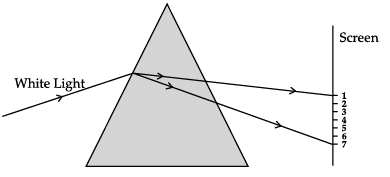
(a) Scattering of light
(b) Dispersion of light
(c) Reflection of light
(d) Refraction of light
Correct Answer is Option (b)
The phenomenon of splitting of white light into its constituent colours after passing through a prism is called as dispersion of light. When the light enters the prism, all the colours have different speeds due to which its gets split into bands.
A narrow beam of white light is passing through a glass prism as shown in the diagram. Study the diagram and answer the following questions.

(a) Formation of rainbow
(b) Twinkling of stars
(c) Blue colour of sky
(d) Advance sunrise
Correct Answer is Option (a)
Rainbow is caused by dispersion of sunlight by tiny water droplets present in the atmosphere which is one of the application of dispersion of light.
A narrow beam of white light is passing through a glass prism as shown in the diagram. Study the diagram and answer the following questions.

(a) White light consists of seven colours.
(b) Violet colour suffers minimum deviation.
(c) Red light suffers maximum deviation.
(d) All the colours of the white light move with different speed in vacuum.
Correct Answer is Option (a)
When the light disperses, various bands of light are clearly visible. It is clear from the figure that the violet light suffers maximum deviation and red light suffers minimum deviation. All the colours of the white light move with the same speed in air or vacuum but with different wavelengths and frequencies.
A narrow beam of white light is passing through a glass prism as shown in the diagram. Study the diagram and answer the following questions.

(a) All the colors of light travel with the speed more than the speed of light.
(b) All the colors have different angles of deviation.
(c) All the colors do not travel with the same speed of light.
(d) All the colors have the same wavelength.
Correct Answer is Option (c)
The various colours of white light have different extent of refraction in a medium. All of colours of light do not travel with same speed in the medium which is the cause of dispersion of light.
|
80 videos|569 docs|80 tests
|