How to prepare for JEE Chemistry with EduRev? | Chemistry for JEE Main & Advanced PDF Download
Chemistry is the most scoring and student-friendly subject in the JEE exam. It consists of three parts – Physical Chemistry, Organic Chemistry and Inorganic Chemistry. With strong preparation in all three sections, it is even possible to score 100 per cent in Chemistry in the JEE exam. The strategy to prepare for the three parts of Chemistry varies and in this document, we will discuss those strategies in detail.
An important tip before we begin - As Chemistry is easier as compared to Physics and Mathematics, some candidates focus less on Chemistry and in turn lose easy marks. Therefore, you should focus equally on Chemistry to get an edge over your competitors and thereby increase your overall JEE score.
How to prepare for Chemistry?
The syllabus and strategy to prepare for chemistry have been prepared by EduRev experts by analysing and compiling the most important tips and strategies for Chemistry followed by JEE mains toppers such as Mridul Agarwal (AIR 1 in JEE advance 2021 and 100 percentile in JEE mains 2021), Amaiya Singhal (100 percentile in JEE mains 2021), Siddhant Mukerjee (100 percentile in JEE mains 2021) and Anmol Arichwal (100 percentile in JEE mains 2021). Our focus in preparing this article has been to provide you with every information that you need to score 100 per cent in the Chemistry part of the JEE exam.
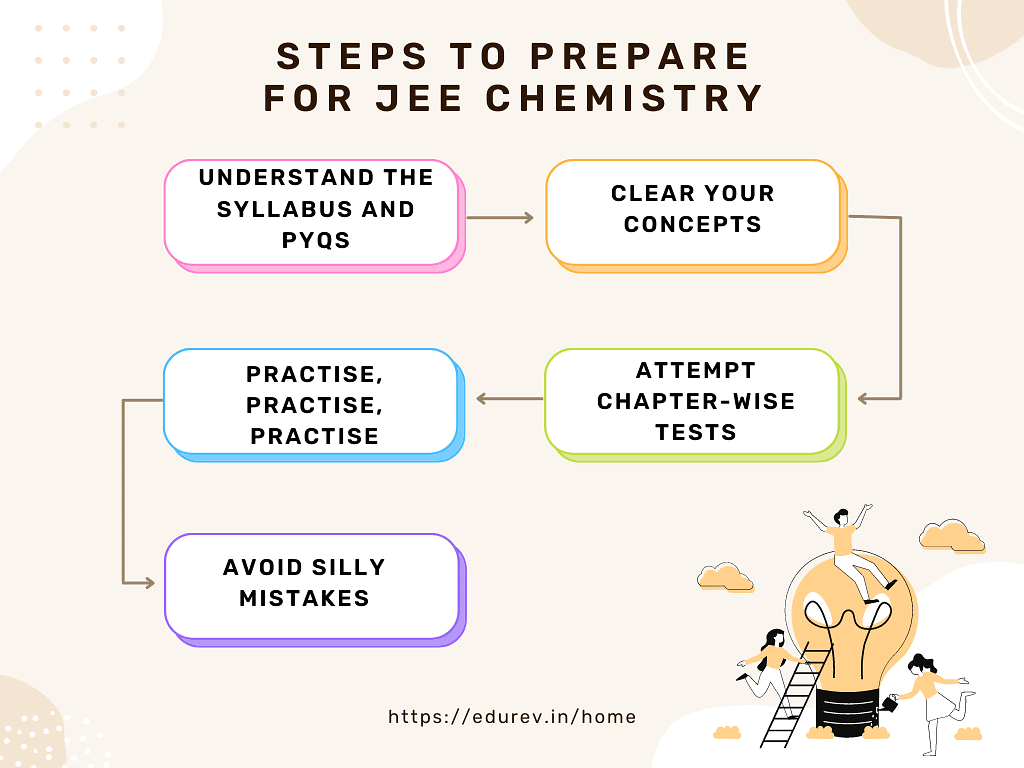
Step 1: Understand the syllabus and PYQs
The JEE syllabus and exam pattern is the Blueprint for the Examination and the first step toward becoming familiar with the exam. Chemistry will have 2 sections in the exam i.e. A and B containing 20 and 10 questions respectively. However, while attempting questions asked in Section B candidates can choose to attempt any 5 Questions out of 10. The complete syllabus for JEE Chemistry can be checked here
The second method to become familiar with the exam pattern is to skim through the Previous Year's Question papers (PYQs). The exam pattern is usually repeated every year. The PYQs for Chemistry JEE can be checked here.
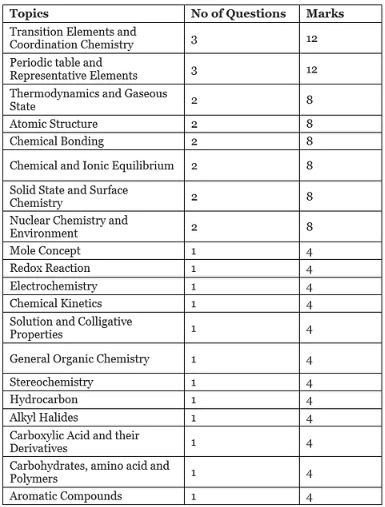
By analysing the PYQs, you can know the topics that interest you the most and the topics that you find difficult. Make a list of the important chapters. The weightage of different chapters is provided at the end of this guide.
If you have understood the syllabus properly then you are not going to waste time and you will actually Study What Matters.
Step 2: Start building your concepts from NCERT.
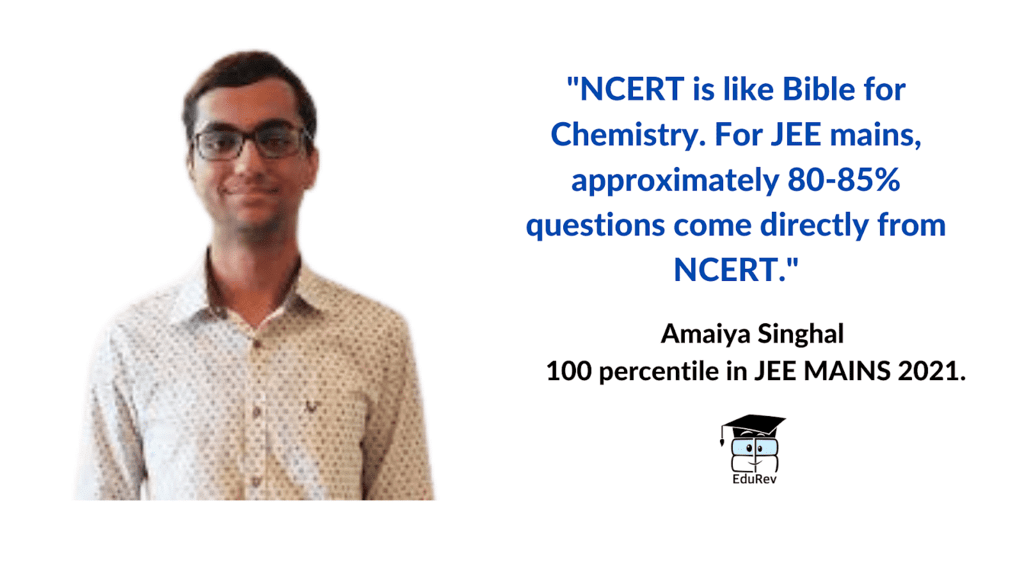
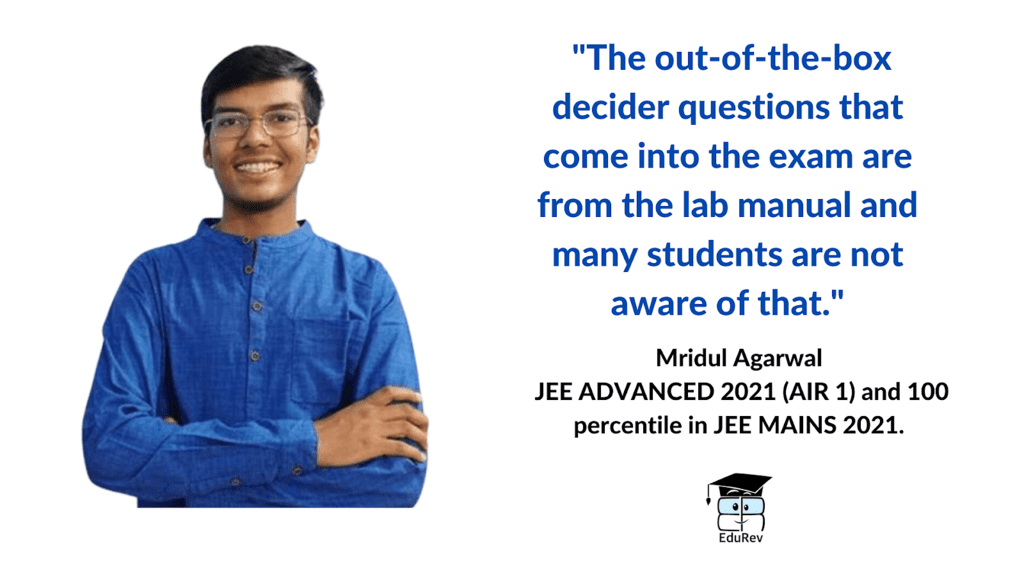
NCERT is the most significant resource for chemistry preparation. It's critical to go over NCERT chemistry line by line. It's also crucial to study the NCERT Lab Manual because certain questions may appear to be off-topic but are actually from the NCERT lab manual. EduRev provides NCERT textbooks, NCERT solutions and NCERT exemplar in each chapter. You can check the NCERT exemplar for class 11 and class 12 available at EduRev.
Learning from a large number of reference books can lead to confusion and the omission of vital topics. As a result, candidates should keep their reference research to a bare minimum.
Instead, focus on making your own notes after each lecture. This will help to improve the clarity of the concept. EduRev also provides tons of revision notes for each chapter of Chemistry.
- EduRev provides chapter-wise mind-maps for class and mind-maps for class 12 which aids in better learning.
Below the strategy to prepare for the sections of chemistry is given.
Physical Chemistry
There are a lot of numerical problems in the Physical Chemistry area.
Solve NCERT and practise questions: Numerical questions, for example, are extremely significant in Physical Chemistry. Before moving on to PYQs, make sure you've completed all of the NCERT in-text and practise questions. EduRev provides solutions to all NCERT questions.
It is critical to have a list of formulas: You should make a list of all the formulae and review it on a regular basis. You should also check the complete formula list available on EduRev of all chapters. Click here to check the link.
NCERT may not be sufficient, thus you need to consult some good works by RC Mukerjee and P. Bahadur. These books can be found on EduRev.
Chapters such as Chemical bonding, Atomic structure, States of Matter, Thermodynamics and Redox Reactions are critical chapters.
Practice tests: After studying each chapter, work through the tests to gain a better understanding of the material.
Inorganic Chemistry
NCERT is Important: For your inorganic chemistry section, NCERT is more than enough. NCERT must be followed; good questions are built from it; no outside questions are asked; only NCERT-based questions are asked.
Essential Chapters: Transition elements and the periodic table make up half of the inorganic chemistry curriculum; thoroughly review them using NCERT. Direct questions are posed in chapters such as the S and P blocks. Environmental Chemistry is the easier chapter. You should memorise their examples.
After studying each chapter, solve the problems to gain a deeper understanding of the idea.
Organic Chemistry
Start with NCERT: You must thoroughly cover the NCERT and take notes in order to revise the chapter from time to time.
Named reactions: It is critical to learn named reactions as well as their processes in organic chemistry.
Tables and charts: According to toppers, you should make short notes in tabular form which makes memorizing easy and effective. The IUPAC names in organic chemistry, periodic table in inorganic chemistry and formulae in physical chemistry are really important and should be at your fingertips. Note them down on paper and keep them handy.
Understand the mechanisms: Rather than memorising the reactions, attempt to grasp the mechanism behind them. You should have access to all of the reactions covered in the NCERT.
Important Subjects: Isomerism, IUPAC, GOC, and O-containing compounds are all essential topics. These topics should be practised more frequently.
After studying each chapter, take a practice exam to see how well you understand the idea.
For concepts and questions, EduRev has compiled a complete course of Chemistry Class 11 & Chemistry Class 12. A complete course Chemistry for JEE is also available with tons of study material. This course provides you with 400+ tests to attempt for complete chapters as well as topic-wise tests are also available. The theory is available in the form of precise docs and videos by the best faculty.
Step 3: Attempt Chapter-wise tests
Kunal Pal, who scored 99.36 percentile in JEE mains 2020 without coaching, followed the strategy of attempting various questions after completing each topic. According to him, even if it would take 1.5-2 hours to solve the questions, he would do it as it was crucial.
You can give chapter-by-chapter and topic-by-topic tests while preparing Chemistry chapters to get a better grasp of your weak and strong areas. Each chapter of EduRev's tests contains roughly 200-300 questions. You can practise concept building and attempt chapter-wise tests by clicking on this link.
Step 4: Practise, Practise, Practise.
Anmol Arichwal said,” I believe that solving previous years’ JEE mains question papers has significantly helped me obtain satisfactory results. Before the JEE Main exam, I solved the question papers of JEE Main 2020 and 2021 (Feb and March) thrice.
It's time to step up your revision game once you've covered the entire curriculum and practised enough questions. You should begin preparing for the exam at least two months ahead of time.
Complete PYQs to determine your weak and strong points. It will also assist you in getting a feel for the final exam and improving your time management abilities. Attempting questions from past years will boost your confidence and help you prepare for the exam. You can find previous 10 years' questions papers on the EduRev platform, click here. As well as you will find Chemistry PYQs of 35 years on our platform, click here.
Attempt simulated tests after completing PYQs. You can get an All India Rank by taking mock tests on EduRev (AIR). Analysing your performance in mock tests and then fixing your weak areas will give your preparation an extra push. Click here for Chemistry mocks.
When you are attempting complete mocks, you will start learning management of time as well as the discipline to sit straight for 3 hours. For complete mocks click here.
You must mark the important and tricky questions while attempting the mocks and go back to them from time to time.
Step 5: Avoid simple errors.
Simple errors are important to avoid to get a good score. As a result, it's critical to avoid making such blunders.
When practising numerical problems, read the question carefully and take careful notes on the numerical figures to prevent making foolish mistakes.
Do not use a calculator when practising because you should develop the habit of doing math on your own. This will increase the speed and accuracy of your calculations.
You have the option of choosing 5 questions out of 10 in section B of the exam. To avoid losing marks, choose questions that need fewer calculations.
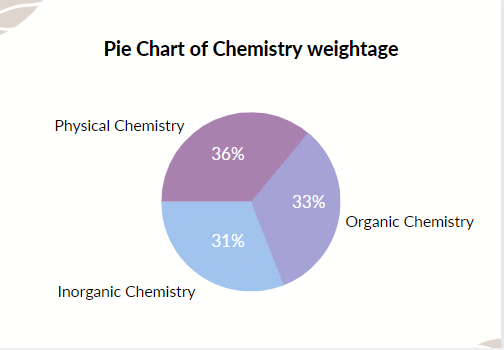
Weightage of JEE mains Chemistry
The complete analysis from the chemistry syllabus is provided below.
The chemistry section of the JEE exam can be further divided into three parts:
Physical Chemistry
The most important topics of Physical Chemistry are:
1. Basic Concepts of Chemistry
Laws of Chemical Combination
Mole concept & Stoichiometry
2. Chemical Bonding & Molecular Structure
Ionic and Covalent Bonding and Bond parameters
VSEPR Theory
Valence bond theory and Hybridization
Molecular Orbital theory
3. States of Matter (Gases, Liquids & Solids)
Gas Laws
Kinetic Molecular Theory of Gases
Real Gas behaviour and Liquefaction of gases
Crystal Lattice and Unit cells
Close packed structures and voids
Defects in Solids
4. Atomic Structure
Dual nature of Radiation
Atomic Spectra
Bohr’s Model for Hydrogen and Hydrogen-like species
Quantum Mechanical Model: Orbitals and Quantum Numbers
5. Solutions
Solutions: Types and Concentration
Vapour Pressure of a solution and Raoult’s law
Colligative properties of Solutions & Van’t Hoff factor
6. Thermodynamics & Thermochemistry
Enthalpy and Heat capacity
Enthalpy change of Various types of reactions
Gibb’s energy and Spontaneity
7. Equilibrium
Law of Chemical equilibrium and equilibrium constant
Factors affecting equilibrium: Le Chatelier’s Principle
Acids, Bases and Concept of pH
Hydrolysis of Salts and Buffer solutions
Solubility and Solubility product of sparingly soluble salts
8. Redox & Electrochemistry
Redox Reactions: Types and Concept of Oxidation Number
Galvanic Cells: features and Electrode Potential
Conductance of Electrolytic Solutions
Electrolytic cells and Products of electrolysis
9. Chemical Kinetics
Rate, Order and Molecularity of a reaction
Integrated rate equations and Half-life
Arrhenius Equation
Radioactivity and Nuclear Reactions (Fission & Fusion)
10. Surface Chemistry
Adsorption: Mechanism, Types and Isotherms
Catalysis
Colloids and Emulsions: Classification and Properties.
Inorganic Chemistry
The most important topics are:
1. Classification of Elements
Modern periodic table and Electronic configuration of Elements
Periodic Trends in Properties of Elements
2. Hydrogen & S Block Elements
Hydrides
Water and Hydrogen peroxide: structure and properties
General Characteristics of Compounds of S block elements
Anomalous Properties of the First Element
Important Compounds of S block elements
3. P Block Elements
General Trends in Physical and Chemical Properties of Elements
General trends in the Properties of Compounds of P block elements
Important Compounds of Boron, Carbon and Silicon
Allotropes and Important Compounds of Nitrogen, Phosphorus and Sulphur
Important Compounds of Halogens and their properties
4. Isolation of Metals
Processes of extraction of Metals from their ores
Thermodynamic and Electrochemical principles of metallurgy
5. D & F Block Elements
General Trends in Physical and Chemical Properties of the Transition Elements
Important compounds of Transition Elements
6. Coordination Compounds
IUPAC Nomenclature and Isomerism of Coordination compounds
Werner’s theory, Valence bond theory & Crystal field theory
7. Environmental Chemistry
Environmental pollution, soil pollution
Organic Chemistry
The most important topics in these chapters are:
1. Basic Principles of Organic Chemistry
Structure and Nomenclature of Organic Compounds
Structural and Stereoisomerism in Organic Compounds
Electronic Displacement Effects in Covalent Bonds
2. Hydrocarbons & Halides
Structure, Physical and Chemical Properties of Alkanes, Alkenes & Alkynes, Benzene, Haloalkanes & Haloarenes
3. Oxygen-containing compounds
Alcohols, Phenols & Ether: Structure, Physical and Chemical Properties
Aldehydes and Ketones: Physical and Chemical Properties
Carboxylic acids and their derivatives: Structure & Chemical properties
4. Nitrogen-containing compounds
Amines & Diazonium salts: Chemical properties
5. Biomolecules
Monosaccharides: Structure, Physical and Chemical properties
Proteins and Enzymes: Structure and properties
Nucleic Acids: Structure and properties
6. Polymers
Addition & Condensation Polymerisation: Mechanism and Examples
Commercial Polymers
7. Chemistry in Everyday life
Drugs: Classification and therapeutic Action
Soaps and Detergents
All three sections of Chemistry have almost equal weightage. According to the trends, Inorganic chemistry usually has the least weightage and Physical chemistry has the most weightage. The weightage of the three sections varies by 5-6% every year.
|
366 videos|829 docs|301 tests
|
FAQs on How to prepare for JEE Chemistry with EduRev? - Chemistry for JEE Main & Advanced
| 1. How can I prepare for Chemistry? |  |
| 2. What is the weightage of Chemistry in JEE Mains? |  |
| 3. How can I prepare for JEE Chemistry with EduRev? |  |
| 4. What are some frequently asked questions about preparing for Chemistry? |  |
| 5. How can I avoid making simple errors in Chemistry? |  |