Notes: Materials | Science & Pedagogy Paper 2 for CTET & TET Exams - CTET & State TET PDF Download
| Table of contents |

|
| Materials |

|
| Properties of Material |

|
| Metals and Non Metals |

|
| Chemical Properties of Metals and Non-Metals |

|
| Applications of Metal and Non-Metal |

|
Materials
Materials are the building blocks of everything around us, forming the objects we use in daily life. They come in different forms and originate from various sources, each imparting unique properties. For example, wood, a natural material, can be used to make both pencils and chairs. The characteristics of a material depend on its source, influencing its strength, durability, and appearance. Understanding materials and their properties helps in selecting the right one for different purposes.
Properties of Material
- Appearance: The appearance of a material can help us identify it. Some materials are shiny, like silver, while others, like wood, are not shiny.
- Hardness: Hardness is another way to classify materials. For example, iron is hard, while cotton is soft.
- Floating Property: Some materials can float in water, while others cannot. For instance, a wooden block can float, but an iron coin cannot.
- Solubility: This property refers to whether a material can dissolve in water. Some materials, like salt, sugar, and lemon juice, are soluble and dissolve in water. Others, like wood, plastic, and oil, are insoluble and do not dissolve in water.
- Transparency:Transparency refers to how much light can pass through a material. There are three types of materials based on transparency:
I) Transparent Materials: These materials allow light to pass through them easily, such as glass sheets, clear water, or thin plastic sheets.
ii) Opaque Materials: These materials do not allow light to pass through, so they cannot be seen through, like wooden tables or Teflon pans.
iii) Translucent Materials: These materials allow some light to pass through, but not enough to see through them clearly, such as butter paper or frosted glass sheets.
Why is the classification of materials necessary?
- Classification is a crucial scientific method that helps us study various subjects with similar properties. For example, understanding the characteristics of wood allows scientists to predict how different wooden objects, like chairs and stools, will behave.
- By classifying materials, we can group similar objects together, making their study easier and more efficient.
Metals and Non Metals
Metals and non-metals are two fundamental categories of materials that make up the world around us. They exist in different forms and possess distinct properties based on their composition and origin. Metals, such as iron and copper, are typically strong, shiny, and good conductors of heat and electricity, making them essential in construction and technology. Non-metals, like oxygen and sulfur, often have varied characteristics, including being brittle, non-conductive, or gaseous. Understanding the differences between metals and non-metals helps in selecting suitable materials for various applications in daily life and industry.
Classification of Metals and Non-Metals
Metals: Metals are materials that possess several key characteristics, which are listed below:
- Conductivity: Metals are excellent conductors of heat and electricity.
- Ductility: They can be stretched into wires.
- Hardness: Most metals are hard; however, sodium and potassium are soft and can be cut with a knife.
- Lustre: Metals have a shiny surface.
- Malleability: They can be hammered into thin sheets.
- Physical State: Most metals are solid at room temperature, with the exception of mercury and gallium, which are liquids.
- Sonorous: Metals produce a ringing sound when struck. Examples include copper, gold, and iron.
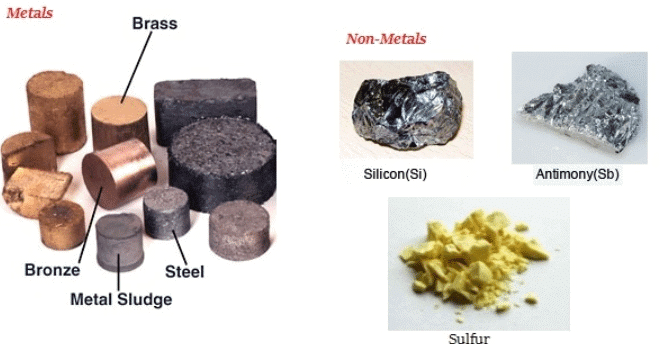
Non-Metals: Non-metals are materials that generally exhibit properties opposite to those of metals.
- Conductivity: Non-metals are poor conductors of heat and electricity, with the exception of graphite, which can conduct electricity due to free electrons.
- Ductility: Non-metals are not ductile; they tend to break under pressure.
- Hardness: Most non-metals are soft, except for diamond, which is extremely hard.
- Lustre: Non-metals are typically dull and lack shine.
- Malleability: Non-metals cannot be hammered into thin sheets.
- Physical State: Non-metals can exist as solids, liquids, or gases at room temperature. Examples include carbon (solid), bromine (liquid), and oxygen (gas).
- Sonorous: Non-metals do not produce ringing sounds when struck.
Chemical Properties of Metals and Non-Metals
1. Reaction of Metals with Oxygen: Metals typically react with oxygen to form metal oxides. For instance:
- Copper + Oxygen → Copper Oxide: 2Cu + O 2 → 2CuO
2. Reaction of Metals with Water: The reaction of metals with water (H 2 O) varies depending on whether the water is in liquid or steam form.
- Liquid Water: When metals react with liquid water, the products are metal hydroxide and hydrogen gas.
- Steam: When metals react with steam, the products are metal oxide and hydrogen gas.
Examples: a. Magnesium + Water: Mg + 2H 2 O → Mg(OH) 2 + H 2 b. Aluminium + Steam: 2Al + 3H 2 O → Al 2 O 3 + 3H 2
3. Reactions of Metals with Solutions of Other Metals: In these reactions, more reactive metals can displace less reactive metals from their compounds in solution.
- Example:Iron + Copper Sulfate: Fe + CuSO 4 → FeSO 4 + Cu In this reaction, iron, being more reactive than copper, displaces copper from copper sulfate.
Applications of Metal and Non-Metal
Metal and Non-Metal are used in many aspects of our daily lives. Here are some important applications:
Uses of Metals
- Machinery: Metals are essential for the production of machinery used in various industries.
- Utensils: Metals are commonly used to manufacture kitchen utensils due to their durability and heat conductivity.
- Electrical Appliances: Metals are a key component in the production of electrical appliances, where they are used for wiring and structural purposes.
- Insulation: Metals are used in insulation to help retain heat in food and beverages, such as in thermos flasks.
- Gadgets and Scientific Equipment: Metals play a vital role in the manufacturing of modern gadgets and scientific instruments due to their strength and conductivity.
Uses of Non-Metals
- Essential for Life: Non-metals like oxygen and carbon dioxide are crucial for the survival of living organisms.
- Fertilizers: Compounds containing nitrogen and phosphorus are commonly used in fertilizers to promote plant growth.
- Medicinal Products: Non-metals are also used in the production of various medicinal products that are essential for healthcare.
|
35 videos|145 docs|32 tests
|
FAQs on Notes: Materials - Science & Pedagogy Paper 2 for CTET & TET Exams - CTET & State TET
| 1. What is the CTET exam? |  |
| 2. How can I apply for the CTET exam? |  |
| 3. What is the significance of clearing the CTET exam? |  |
| 4. What is the syllabus for the CTET exam? |  |
| 5. What is the validity of the CTET certificate? |  |




















