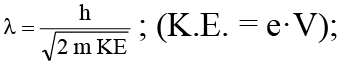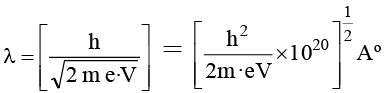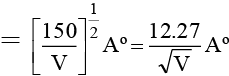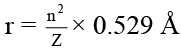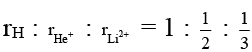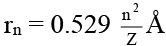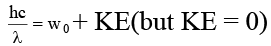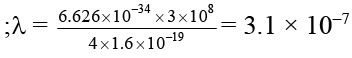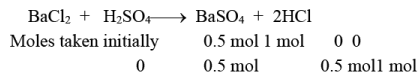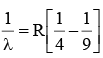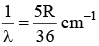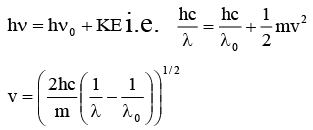JEE Advanced (Single Correct Type): Structure of Atom | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. The wave function ψ of 2s – orbital is given by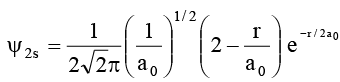
At r = r0, radial node is formed. Which of the following is correct?
(a) r0 = 2a0
(b) 2r0 = a0
(c) r0 = 3a0
(d) None of these is correct
Correct Answer is option (a)
At r = r0 ψ 2s 2 = 0
It is possible only when 2 - r/a0 = 0
∴ r0 = 2a0
Q.2. Which of the following set of quantum numbers belong to highest energy?
(a) n = 2, l = 1, m = 0, s = + 1/2
(b) n = 3, l = 0, m = 0, s = + 1/2
(c) n = 4, l = 0, m = 1, s = + 1/2
(d) n = 4, l = 1, m = 1, s = + 1/2
Correct Answer is option (d)
For (d), the value of n + l = 3 + 2 = 5.
In other cases the value of (n + l) is less than 5.
The orbital having higher (n + l) value has higher energy.
Q.3. An electron is accelerated through V voltage. Its de Broglie wavelength is
(a) 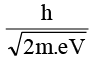
(b) 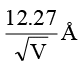
(c) 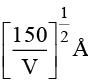
(d) All of these
Correct Answer is option (d)
Q.4. Which one among the following statements is correct?
(a) An orbital containing an electron having quantum numbers n = 2; l = 0; m= 0 and s = +1/2 is spherical.
(b) The frequency of X-rays is less than that of radio waves.
(c) All photons have same energy.
(d) As intensity of light increases, its frequency also increases.
Correct Answer is option (a)
Any orbital with l = 0 has spherical symmetry irrespective of the value of its principal quantum number.
Q.5. The ratio of radii of first orbits of H, He+ and Li2+ is
(a) 1: 2: 3
(b) 6: 3: 2
(c) 1: 4: 9
(d) 9: 4: 1
Correct Answer is option (b)
= 6 : 3 : 2
Q.6. How many electrons will have m (magnetic quantum number) = 0 in Fe3+ ion?
(a) 12
(b) 13
(c) 11
(d) 14
Correct Answer is option (c)
Fe: 1s22s22p63s23p64s23d6
Fe3+: 1s22s22p63s23p63d5
Electron with m = 0 are 11.
Q.7. The radius of second stationary orbit in Bohr’s atom is R. The radius of the third orbit will be
(a) 3 R
(b) 9 R
(c) 9/4 R
(d) 4 R
Correct Answer is option (c)
(for n = 2)
(for n = 3)
Q.8. The work function for a metal is 4 eV. To emit a photoelectron of zero velocity from the surface of the metal, the wavelength of incident light should be
(a) 2700 Å
(b) 1700 Å
(c) 5900 Å
(d) 3100 Å
Correct Answer is option (d)
m = 3100 Å.
Q.9. The maximum amount of BaSO4 that can be obtained on mixing 0.5 mol BaCl2 with 1 mol H2SO4 is
(a) 0.5 mol
(b) 0.1 mol
(c) 0.15 mol
(d) 0.2 mol
Correct Answer is option (a)
The limiting reagent is BaCl2. Hence, a maximum of 0.5 mole of BaSO4 will be obtained.
Q.10. The wave number of the first emission line in the H–atom spectrum in the Balmer series in terms of Rydberg’s constant (RH) is given by
(a) 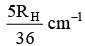
(b) 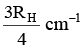
(c) 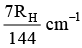
(d) 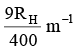
Correct Answer is option (a)
n1 = 2, n2 = 3, Z = 1
Q.11. It ‘λ0' is the threshold wavelength for photoelectric emission, ‘λ' the wavelength of light falling on the surface of a metal and 'm' is the mass of the electron, then the velocity of ejected electron is given by
(a) 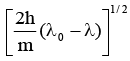
(b) 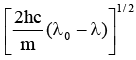
(c) 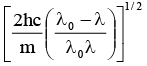
(d) 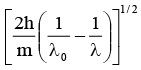
Correct Answer is option (c)
Q.12. Energy of the third orbit of Bohr’s atom is
(a) -13.6 eV
(b) -34 eV
(c) -1.5 eV
(d) None of the three
Correct Answer is option (c)
[Z = 1, n = 3]
Q.13. The electronic velocity in the fourth Bohr’s orbit of hydrogen is V. The velocity of the electron in the first orbit would be:
(a) 4 V
(b) 16 V
(c) V/4
(d) V/16
Correct Answer is option (a)
Q.14. Which of the following statement is correct in relation to the hydrogen atom?
(a) 3s–orbital is lower in energy than 3p–orbital.
(b) 3p–orbital is lower in energy than 3d–orbital.
(c) 3s and 3p–orbitals are of lower energy than 3d–orbitals.
(d) 3s, 3p and 3d–orbitals all have same energy.
Correct Answer is option (d)
Energy of single electron system is only depend on the principle quantum number, so that energy of different orbitals of same principle quantum number is same.
Q.15. The outermost electronic configuration of the most electronegative element is
(a) ns2np3
(b) ns2np4
(c) ns2np5
(d) ns2np6
Correct Answer is option (c)
|
481 docs|964 tests
|