JEE Advanced (One or More Correct Option): Chemical Kinetics & Nuclear Chemistry | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Which of the following statement(s) is/are correct?
(a) Densities of diamond and graphite are x and y (x > y). Increase in pressure on the equilibrium favours backward reaction. C (diamond) ⇌ (graphite).
(b) An increase in temperature increases the rate constant as well as the equilibrium constant of an exothermic reaction.
(c) For the reaction N2O4(g) ⇌ 2NO2(g), if degrees of dissociation of N2O4 are 25%, 50% and 75%, then gradation of observed molar masses is M1 > M2 > M3 (M1, M2 and M3 are molar masses corresponding to vapour densities at 25%, 50% and 75% dissociation respectively)
(d) Solubility of a solute (s) is dependent upon temperature as S = A·e–ΔH/RT (Here ΔH is the enthalpy of solution).
Correct Answer is option (a, c, d)
For (A), On increase in pressure in physical equilibria, the equilibrium shifts in the direction of increasing density.
For (B), On increase in temperature, rate constant increases. However, on increase in temperature, Keq decreases in exothermic reactions and increases in endothermic reactions.
For (C)
Q.2. The catalyst provides a new pathway involving lower amount of activation energy, to make the reaction fast. Which one of the following is/are the result of catalytic action.
(a) Catalyst decreases Ea/RT value.
(b) Catalyst increases –Ea/RT value.
(c) Catalyst increases eEa / RT value.
(d) Catalyst increases k of reaction
Correct Answer is option (a, b, d)
To increase the rate of reaction catalyst does
(i) decrease Ea
(ii) decreases Ea/RT
(iii) increases –Ea/RT
(iv) increases e-Ea/RT
(v) increases k
Q.3. Which isotopes emit positrons?
(a) 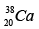
(b) 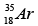
(c) 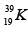
(d) 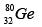
Correct Answer is option (a, b, c)
n /p <1 , positron emission occurs.
More protons are there, so positron emission occurs.
Q.4. 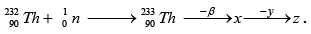
Pick out the correct statements
(a) x is protactinium
(b) If y is a β - particle, then z is 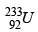
(c) x is a fissile isotope and used as a popular nuclear fuel
(d) This is a good scheme to convert non fissile isotope into fissile isotope.
Correct Answer is option (a, b, d)
Q.5. The polarimeter readings in an experiment to measure the rate of inversion of cane sugar (1st order reaction) were as follows: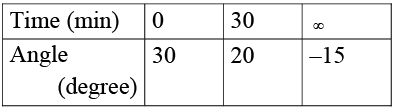 Identify the true statement (s)? [log 2 = 0.3, log 3 = 0.48, log 7 = 0.84, loge 10 = 2.3]
Identify the true statement (s)? [log 2 = 0.3, log 3 = 0.48, log 7 = 0.84, loge 10 = 2.3]
(a) The half-life of the reaction is 75 min
(b) The solution is optically inactive at 120 min
(c) The equimolar mixture of the products is dextrorotatory
(d) The angle would be 7.5° at half time
Correct Answer is option (a, b, d)
Let's assume specific, angle of rotation of sucrose, glucose and fructose be rs, rg & rf respectively.
at t = 0 (a) (rs) + (0) (rg) +(0)(rf) = 30
at t = 30
(a – x) (rs) + (x) (rg) + (x) (rf) = 20
at t = ∞
(0) (rs) + (a) (rg) + (a) (rf) = –15
On solving, we get:
On calculatingthe problem can be solved.
Q.6. An autocatalytic reaction is represented as A + B → D where initial concentrations of A and B each equal to a0. initial concentration of D is zero-
(a) An autocatalytic reaction is the such reaction in which product itself increases the speed of reaction.
(b) In an autocatalytic reaction product form a complex of lower activation energies.
(c) The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3.
(d) The overall order of the above reaction is three.
Correct Answer is option (a, c, d)
∴ at the maximum rate, dr/dx = 0
or 3x (x – a0) – a0 (x – a0) = 0
or (3x – a0) (x – a0) = 0
∴ x = a0 or x = a0/3
But x ≠ a0 ∴ x = a0/3
Q.7. For two parallel path reaction the rate constants and Ea are shown: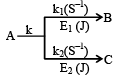 Select the correct statement.
Select the correct statement.
(a) Arrhanius constants for both the process may have different values
(b) The overall energy of activation 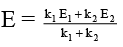
(c) [B] = k1/k [A0] (1 – e–kt)
(d) [B]/[C] = k1/k2
Correct Answer is option (a, b, c, d)
- Since rate constants are different so A may have different value.
- For any parallel path first order reaction

∴ [A] = [A0] e–Rt
dB/dt = K1 [A] = K1 [A0] e–Rt
on integration,
Q.8. Rate of chemical reaction 2A(g) → B(g) is defined as rB = 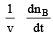 where nB = no. of moles of B formed & CB = conc. of B, which of the following relation is correct -
where nB = no. of moles of B formed & CB = conc. of B, which of the following relation is correct -
(a) 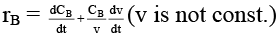
(b) 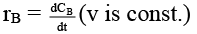
(c) 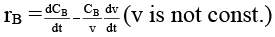
(d) 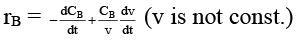
Correct Answer is option (a, b)
case (i) If V is constant
case (ii) If V is not const.
Q.9. The rate constant of a reaction is given by 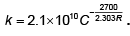 It means that
It means that
(a) log k vs 1/T will be straight line with slope 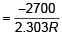
(b) log k vs 1/T will be a straight line with intercept on log k axis = log 2.1 × 1010.
(c) the number of effective collisions are 2.1 × 1010 cm-3 sec-1
(d) half-life of the reaction increases with increase in temperature
Correct Answer is option (a, b)
(a) and (b) are correct, (c) is wrong because frequency factor gives total number of collisions and not the effective collision cm–3 sec–1, (d) is wrong because half-life of the reaction decreases with increase in temperature (as reaction becomes faster).
Hence, (a) and (b) are correct answer.
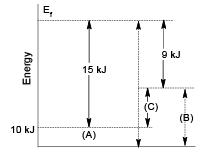
Q.10. In a hypothetical reaction A → B, the activation energies for the forward and backward reactions are 15 and 9 kJ/mol respectively. The potential energy of A is 10 kJ/mol, then
(a) the threshold energy of the reaction is 25 kJ/mol
(b) potential energy of B is 16 kJ
(c) heat of reaction is 6 kJ
(d) the reaction is endothermic
Correct Answer is option (a, b, c, d)
Hence, (a), (b), (c) and (d) are correct answer.
|
446 docs|930 tests
|

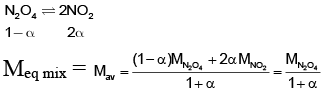
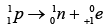
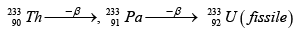
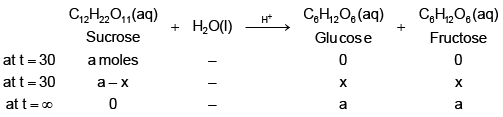
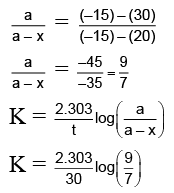
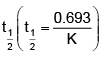 the problem can be solved.
the problem can be solved.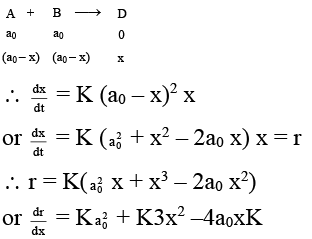
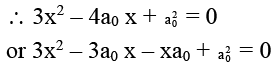
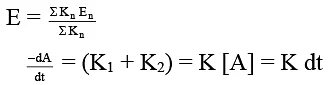
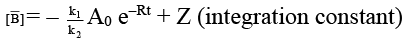
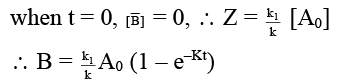
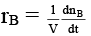
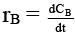
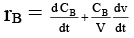
 Hence, (a), (b), (c) and (d) are correct answer.
Hence, (a), (b), (c) and (d) are correct answer.



















