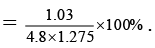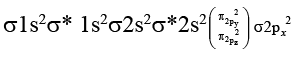JEE Advanced (Single Correct Type): Chemical Bonding & Molecular Structure | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Which one of them is the weakest?
(a) Ionic bond
(b) Covalent bond
(c) Metallic Bond
(d) van der Waals force
Correct Answer is option (d)
Among them van der Waals force is the weakest force.
Q.2. In the resonating structures of benzene, the number of sigma and pi bonds are
(a) 3π and 12σ
(b) 3σ and 3π
(c) 6σ and 6π
(d) 12σ and 12π
Correct Answer is option (a)
In Benzene (C6H6) ring contain 3 alternative double bond = 3π bonds and (six C-C sigma bond + six C-H sigma bond) = 12 σ bonds
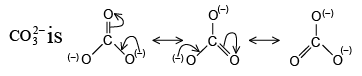
Q.3. The ion which is iso-electronic with CO is______
(a) CN–
(b) O2–
(c) N2+
(d) O2+
Correct Answer is option (a)
Both CO (6 + 8=14) and CN– (6 + 7 + 1 = 14) have the same electrons. so they are iso-electronic with each other.
Q.4. Which of the following molecules have trigonal planar geometry?
(a) BF3
(b) NH3
(c) PCl3
(d) IF3
Correct Answer is option (a)
BF3 has trigonal planar geometry. The hybridization of BF3 is sp2 hybridization.
Q.5. In which of the following pairs, the two molecules have identical bond orders:
(a) N2 , O22+
(b) N2 , O2–
(c) N2– , O2
(d) O22- , N2
Correct Answer is option (a)
Both N2 and O22+ have bond order equal to 3.0.
Q.6. The bond angle around atom which uses sp2 hybridization is ———–
(a) 1200
(b) 1800
(c) 1070
(d) 1090. 28’
Correct Answer is option (a)
The bond angle around an atom which uses sp2 hybridization is 1200.
Q.7. Which of the following substances has a dipole moment more than zero?
(a) Water
(b) Methane
(c) Carbon dioxide
(d) Nitrogen
Correct Answer is option (a)
The dipole moment (μ) of H2O = 1.84 D
Q.8. The correct bond order in the following species is —————.
(a) O2+ < O2– < O22+
(b) O2– < O2+ < O22+
(c) O22+ < O2+ < O2–
(d) O22+ < O2– < O2+
Correct Answer is option (b)
The correct one is O2– < O2+ < O22+ , bond order is 1.5 < 2.5 < 3.0 respectively.
Q.9. During change of O2 to O22- ion, the electron adds on which of the following orbitals?
(a) σ* orbital
(b) π orbital
(c) σ orbital
(d) π* orbital
Correct Answer is option (d)
The incoming electrons added to π* orbital in the change of O2 to O22- ion.
Q.10. Ionic bonds will be formed more easily between elements with comparatively:
(a) low ionization enthalpy and high electron affinity
(b) high ionization enthalpy and high electron affinity
(c) low ionization enthalpy and low electron affinity
(d) high ionization enthalpy and low electron affinity
Correct Answer is option (a)
Ionic bonds will be formed more easily between elements with low ionization enthalpy and high electron affinity.
Q.11. The bond angle is minimum in
(a) NH+4
(b) NOCl
(c) H2Se
(d) SO3
Correct Answer is option (c)
hybridisation – 109°28'.
NOCl → sp2 hybridisation - 120°
H2Se → sp3 hybridisation because of lone pair bond angle gets minimized.
SO3 → sp2 hybridisation 120°.
Q.12. The observed dipole moment of HCl molecule is 1.03 D. If H–Cl bond distance is 1.275 Å and electronic charge is 4.8 × 10–10 e.s.u. The % polarity in HCl will be
(a) 1.275 × 1.03 %
(b) 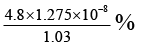
(c) 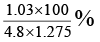
(d) 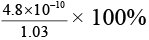
Correct Answer is option (c)
Percentage polarity
Here observed dipole moment of HCl = 1.03 D
Calculated dipole moment of HCl = 4.8 × 10-10 × 1.275 × 10-8
= 4.8 × 1.275 D
Therefore, % polarity
Q.13. Which of the following has same bond order as NO+ has?
(a) CN–
(b) O2–
(c) CN+
(d) none of them
Correct Answer is option (a)
NO+ and CN- both have 14 electrons and also both will have the following configuration.
∴ Bond order of NO+ = Bond order of CN- = 3.
Q.14. The correct order of increasing bond angles is
(a) PF3 < PCl3 < PBr3 < PI3
(b) PI3 < PCl3 < PBr3 < PF3
(c) PI3 < PBr3 < PCl3 < PF3
(d) PCl3 < PBr3 < PI3 < PF3
Correct Answer is option (a)
As the size of surrounding atom increases the electronic repulsion increases bond angle increases.
Q.15. Which of the following is planar?
(a) XeO4
(b) XeO2F2
(c) XeO3F2
(d) XeF4
Correct Answer is option (d)
XeO4 - tetrahedral
XeO3F2 - trigonal bipyramidal
XeO2F2 - trigonal bipyramidal
XeF4 - square planar.
Q.16. Which of the following does not contain coordinate bond?
(a) 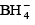
(b) 
(c) 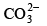
(d) H3O+
Correct Answer is option (c)
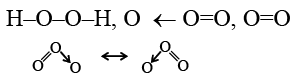
Q.17. The correct order in which the O–O bond length increases in the following is
(a) O2 < O3 < H2O2
(b) H2O2 < O3 < O2
(c) O3 < O2 < H2O2
(d) O2 < H2O2 < O3
Correct Answer is option (a)
Due to resonance in O3, O–O bond length will be in between O=O and O–O.
Q.18. Which species has the maximum number of lone pair of electrons on the central atom?
(a) 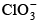
(b) XeF4
(c) SF4
(d) 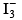
Correct Answer is option (d)
Incentral atom has 3 lone pair and two bond pair.
Q.19. Which of the following compound has highest covalent character?
(a) NaCl
(b) KCl
(c) MgCl2
(d) LiCl
Correct Answer is option (c)
More is the charge on cation, more is the polarisation of anion by cation. Hence, more is the covalent character.
Q.20. Which one of the following species is paramagnetic?
(a) 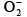
(b) CN–
(c) CO
(d) NO+
Correct Answer is option (a)
Onlyhas unpaired electrons in molecular orbital.
|
481 docs|964 tests
|