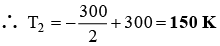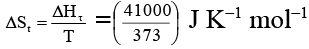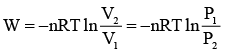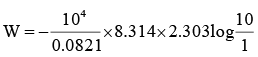Integer Answer Type Questions for JEE: Thermodynamics | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. One mole of an ideal gas is allowed to expand reversibly and adiabatically from a temperature of 27°C. If the work done during the process is 3 kJ, the final temperature will be equal to (Cv = 20 JK–1)
Ans. 150 K
dW = dU
nCVΔT = 3
1 × 20 × ΔT = 3 × 103(Since after expansion the temperature will be lowered)
Q.2. Enthalpy change for the transition of liquid water to steam is 41 kJ mol–1 at 100ºC. The entropy change for the above process is
Ans. 110
= 110 J K–1 mol–1
Q.3. 1m3 of neon gas initially at 273.2 K and 10 atm undergoes expansion isothermally and reversibly to final pressure of 1 atm. The work done by the gas is
Ans. –2332 kJ
Work done in isothermal reversible process is
So, to apply the above equation first we calculate ‘n’ by using PV= nRT
So,
W = –2332 kJ
Q.3. A piston exerting a pressure of 1 bar rests on the surface of water at 100ºC. The pressure is reduced to a small extent and as a result 10 g of water evaporates and absorb 20.0 kJ of heat. The value of ΔHvap. of water is
Ans. 36 kJ mol–1
Heat absorbed by 10 g of water = 20.0 kJ
Heat absorbed by 18 g of water = 20.0 × 18/10 = 36 kJ
∴ ΔHvap of water = 36 kJ mol-1.
Q.4. The dissociation energy of CH4 is 360 kcal/mol and of ethane is 620 kcal/mol. Calculate C-C bond energy.
Ans. 80 kcal
Given, CH4 → C + 4H ;
∴ Bond energy of 4(C-H) bond = 360 kcal
∴ Bond energy of C-H bond = 360/4 = 90 kcal
In C2H6 → 2C + 6H
Bond energy of C2H6 = 1(C-C) + 6(C-H)
∴ 620= 1(C-C) + 6 × 90= 80 kcal
Bond energy of C-C = 80 kcal.
Q.5. Compute the magnitude of heat of formation of liquid methyl alcohol in kilo joule per mol using the following data. Heat of vaporisation of liquid methyl alcohol = 38 kJ/mol. Heat of formation of gaseous atoms from the elements in their standard states: H, 218 kJ/mol; C, 715 kJ/mol ; O, 249 kJ/mol.
Average bond energies: C-H, 415 kJ/mol C-O, 356 kJ/mol O-H, 463 kJ/mol.
Ans. -266 kJ mol-1
C(s) + 2H2(g) + ½O2(g) → CH3OH(l) ; ΔH = ?
ΔH = Bond energy data for formation + bond energy data for dissociation + energy released during liquefaction
= -[3C-H + 1C-O + 1O-H] + [Cs → g + 2H-H + ½O-O]
- [CH3OH(g) → CH3OH(l)]
= -[3 × 415 + 356 + 463] + [715 + 2 × 436 + ½ × 249] - 38
= -266 kJ mol-1.
|
446 docs|929 tests
|