What is the Finkelstein Reaction?
Alkyl halide (alkyl bromide or alkyl chloride) when combined with metal iodide reacts in the presence of a polar aprotic solvent, i.e., acetone which results in precipitation in the form of metal halide salt and alkyl iodide. This reaction is known as the Finkelstein reaction. Replacement of halogen through sn2 Reaction happens when primary alkyl halide is treated with an alkali metal halide.
The chemical equation of the Finkelstein reaction is
 where, X= Cl, Br
where, X= Cl, BrThe reaction between bromide, Nal, alkyl chloride, and dry acetone results in alkyl iodides. The chemical equation for this reaction is:
CH3CH2-Br + NaI → CH3CH2-I + NaBr
Ethyl Bromide + Sodium Iodide (in presence of acetone) → Ethyl Iodide + Sodium Bromide
 Alkyl iodide can be formed from acids if red mercuric and iodine are used. Esters and alkyl halides can be formed from the aliphatic carboxylic acids. The chemical equation of such a reaction is:
Alkyl iodide can be formed from acids if red mercuric and iodine are used. Esters and alkyl halides can be formed from the aliphatic carboxylic acids. The chemical equation of such a reaction is:
RCOOH + R (COO)4Pb → X2 → RCOOR + 2RX
Through halogen exchange, two types of reactions take place: Finkelstein Reaction and Swarts Reaction. Swarts Reaction is the best way to produce alkyl fluorides by heating bromide or alkyl chloride in the presence of AgF, Hg2F2, CoF2, or SbF3 which are also known as metallic fluoride.
Conditions for Finkelstein Reaction
The Finkelstein reaction will not take place if we use metal bromide or metal chloride as a substitute for metal iodide and alkyl iodide is used in the place of alkyl bromide or alkyl chloride because of certain reasons:
- Acetone can dissolve covalent compounds in it as a polar aprotic solvent which can be considered as a covalent compound.
- NaCl, Nal, NaBr along with all metal halides are ionic compounds. A higher covalent compound can be formed when there is an involvement of a large anion and a small cation.
- The cation Na+ is unchanged in NaCl and Nal but anion Cl-, Br- and l- in NaCl, Nal, and NaBr changes its size.
- Nal displays the highest covalent character as anion I- (iodide) is larger. Nal gets easily dissolved in acetone whereas the other two metal halides which are NaBr and NaCl remain insoluble in acetone.
Thus, the Finkelstien reaction depends on several conditions such as :
- Carbon halogen bond
- Nature of group
- Alkyl halide reactivity
- Nucleophilicity
How does a Finkelstein reaction work?
Finkelstein Reaction is a simple and easy process that maintains equilibrium. The insolubility of the newly formed metal halide salt in acetone supports the forward reaction. The reaction is known as a single step bimolecular nucleophilic substitution reaction which is also known by the name of an SN2 reaction. The SN2 reaction happens with the alteration of stereochemistry.
Aromatic finkelstein reaction
Bromides and aromatic chlorides are not easily replaced by iodide, rather it can only be replaced if catalyzed properly. Cul copper(I) iodide when mixed with diamine ligands is the only ideal catalyzed process for Finkelstein reaction. Aromatic Finkelstein reactions can also take place with the help of nickel bromide and tri-n-butylphosphine.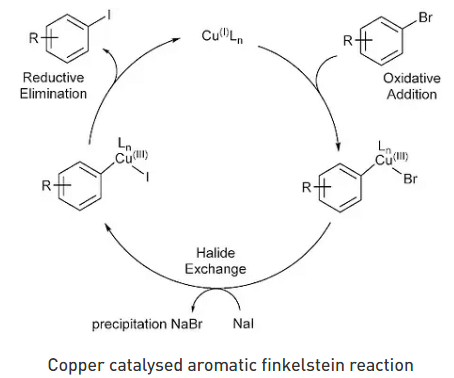
Advantages of Finkelstein Reaction
The advantages of the finkelstein reaction are:
- Suitable production of alkyl iodide.
- The Nal in acetone can be used as a qualitative test to detect the class of an anonymous alkyl halide.
- Alkyl halides excessively vary in the capacity with which they undergo the Finkelstein reaction which is why it is used for analysis.
Examples of Finkelstein Reaction
The reaction between sodium iodide and methyl bromide
- CH3Br + NaI → CH3I + NaBr
The reaction between sodium iodide and ethyl bromide
- CH3CH2Br + NaI → CH3CH2I + NaBr
The reaction between sodium iodide and ethyl chloride
- CH3CH2Cl + NaI → CH3CH2I + NaCl
Things to Remember
- The Finkelstein reaction shows the exchange of one halogen for another.
- The reversible reaction of halide exchange occurs because of the varied Solubility of metal halide salts in acetone solvent
- There is an increase in the rate of reaction of the electron donors on the alkyl halide whereas the rate decreases for the electron-withdrawing groups.
- To achieve the desired alkyl halide in the modified version of the Finkelstein reaction, alcohol is initially converted into a mesylate or tosylate and then treated with metal halide. Mesylate and tosylate are appropriate withdrawing groups which is why the reaction works perfectly.
- Catalysts such as FeCl3, ZnCl2, and many more in CS2 solvent are used to increase the reactivity of secondary, tertiary, vinyl, and aryl halides which are less reactive in Finkelstein reaction.
- Cul copper (I) iodide when mixed with diamine ligands is the only ideal catalyzed process for aromatic Finkelstein reaction.



















