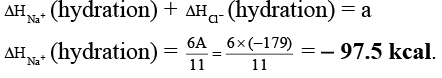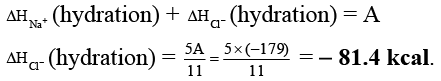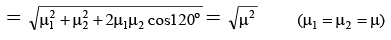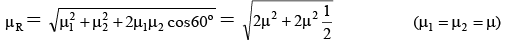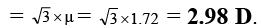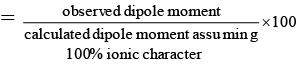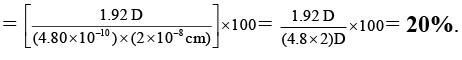Integer Answer Type Questions for JEE: Chemical Bonding & Molecular Structure | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. If MX3 is T shaped, then the number of lone pair around M is
Ans. 2
For T-shape 2 lps appear at same side of axial line at plane of paper.
Q.2. The lattice energy of solid NaCl is 180 kcal/mol. The dissolution of the solid in water in the form of ions is endothermic to the extent of 1 kcal/mol. If the solution energies of Na+ and Cl- are in the ratio 6:5, what is the enthalpy of hydration of Na+ ion?
Ans. -97.5
Na+(g) + Cl-(g) → NaCl(s) ; ΔH = 180 kcal
NaCl(s) + aq → Na+(aq) + Cl-(aq) ; ΔH = 1 kcal
Na+(g) + Cl-(g) + aq. → Na+(aq.) + Cl- (aq.) ; ΔH = A kcal
ΔHsolution = ΔHlattice + ΔHhydration
∴ 1 = 180 + ΔHhydration
∴ ΔHhydration = - 179 kcal = A
Q.3. The lattice energy of solid NaCl is 180 kcal/mol. The dissolution of the solid in water in the form of ions is endothermic to the extent of 1 kcal/mol. If the solution energies of Na+ and Cl- are in the ratio 6:5, what is the enthalpy of hydration of Cl- ion?
Ans. -81.4
Na+(g) + Cl-(g) → NaCl(s) ; ΔH = 180 kcal
NaCl(s) + aq → Na+(aq) + Cl-(aq) ; ΔH = 1 kcal
Na+(g) + Cl-(g) + aq. → Na+(aq.) + Cl- (aq.) ; ΔH = A kcal
ΔHsolution = ΔHlattice + ΔHhydration
∴ 1 = 180 + ΔHhydration
∴ ΔHhydration = - 179 kcal = A
Q.4. A molecule MX4 has a square planar shape. The number of non-bonding pair of electrons is
Ans. 2
Q.5. The dipole moment (in Debye units) of m-dichlorobenzene is 1.72. What is the value of dipole moment for o- dichlorobenzene?
Ans. 2.98
μR =
μ = μR = 1.72
Q.6. A diatomic molecule has a dipole moment of 1.92 D and a bond length of 2.0Å. What is the percentage ionic character in the molecule if e = 4.8 × 10–10 esu?
Ans. 20
% ionic characters
Q.7. If the electronegativity difference between two atoms A and B is 2.0, then the percentage ionic character in the molecule is
Ans. 46
% ionic character = 16(ΔE.N) + 3.5(ΔE.N)2
= 16 (2) + 3.5 (2)2
= 46
Q.8. The bond of CN+
Ans. 2
Total e- are 6 + 7 - 1 = 12e-
Bond order of CN+ = 4-0/2 = 2
Q.9. The number of unpaired electrons in N+2
Ans. 1
N+2 total number of e- are 13e-
Only one electron is unpaired.
Q.10. Maximum number of Hydrogen bond formed by one molecule of H2O is.
Ans. 4
In Ice maximum number of hydrogen bonding by H2O molecule are four.
|
446 docs|929 tests
|