What is Rosenmund Reduction?
Acid chlorides(R-CO-Cl) are converted into aldehydes(R-CHO) by undergoing hydrogenolysis.
In rosenmund reduction hydrogen gas(H2) is passed through palladium(Pd) on barium sulfate(BaSO4). Tertiary amine(R3N) is required to control the activity of the catalyst as well as to neutralize the HCl produced during the reaction (and prevent over reduction). Barium sulfate has less surface area; it restricts the activity of palladium. Thus, it reduces the ability of palladium to react in order to prevent over- reduction of acid chloride. Therefore BaSO4 or CaCO3 act as a support by facilitating easy escape of the product to prevent over-reduction. With the addition of poison, the activity is further diminished for the more reactive acyl chlorides. To avoid excessive hydrogenation, a toxin like thioquinanthrene or thiourea is utilised. If the deactivation does not occur, it could result in additional aldehyde reduction and the production of primary alcohol. If this primary alcohol is created, it will subsequently interact with the residual acyl chloride to generate ester.
Mechanism of Rosenmund Reduction
Step 1: Insertion
Pd gets inserted between C-Cl bond.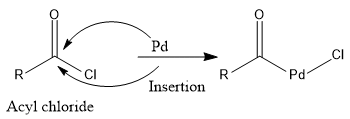
Step 2: Oxidative addition
H2 adds to Pd.
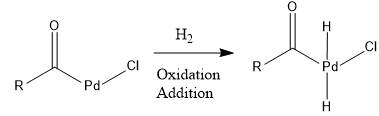
Step 3: Reductive elimination
Pd and HCl gets eliminated to give aldehyde as the final product.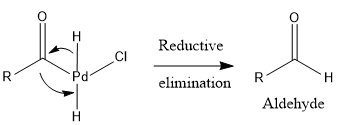
Uses of Rosenmund reduction
- Rosenmund reduction is used in the synthesis of saturated fatty aldehydes.
- It is also useful in manufacturing alkyl halides and aryl halides.
Limitations of Rosenmund reduction
- This reduction method cannot be used in the preparation of formaldehyde. This is because formyl chloride formed upon reduction, is highly unstable at room temperature.














