Introduction
It is a reaction of organic compounds having an aldehydic group (-CHO). It was given by Stanislao Cannizzaro in 1853 and hence was named after him. We have already studied the aldol condensation which is also an organic compound reaction having an aldehydic group which contains α-hydrogen in presence of dilute alkali giving β-hydroxy aldehyde as condensation product. The product contains hydroxy (-OH) and aldehyde (-CHO) group, hence it is known as Aldol condensation. The Cannizzaro reaction differs from the aldol with respect to the α-hydrogen.
Requirements for the Cannizzaro Reaction
- Aromatic aldehydes which do not contain α-hydrogen, for example, C6H5CHO (benzaldehyde)
- Aliphatic aldehydes which do not contain α-hydrogen, for example, HCHO, (CH3)3CCHO
- Presence of concentrated aqueous alkali (KOH, NaOH)
Products formed are:
- Reactants undergo self-oxidation and reduction to give alcohol and the salt of the corresponding carboxylic acid.
Reaction involved: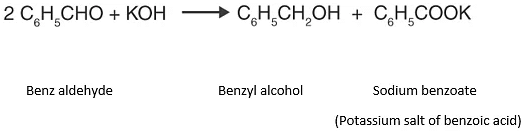
 This disproportionation reaction (same reactant undergoing oxidation and reduction reaction) of aldehydes without α-hydrogen is known as Cannizzarro reaction.
This disproportionation reaction (same reactant undergoing oxidation and reduction reaction) of aldehydes without α-hydrogen is known as Cannizzarro reaction.
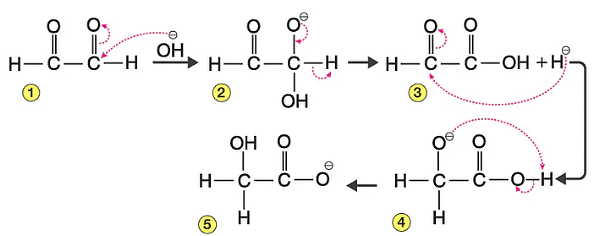
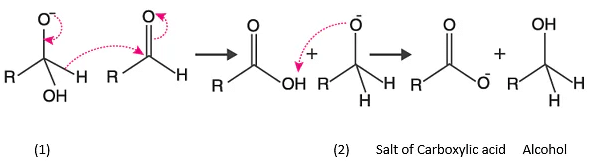
The mechanism for the reaction:
Step 1:
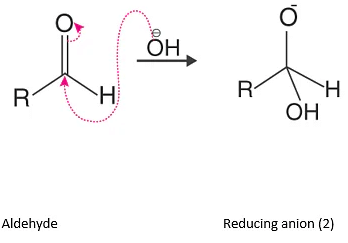
In this step OH–(hydroxide ion), which is a nucleophile and attack on carbonyl carbon of aldehydic group to give reducing anion (1).
 Step 2:
Step 2:
- The anion formed in the first reaction transfers a hydride ion to carbonyl carbon of another molecule of the same aldehyde as used in step 1, forming carboxylic acid and the alkoxide ion.
- The shift of proton from acid to (2) gives the final product as alcohol.
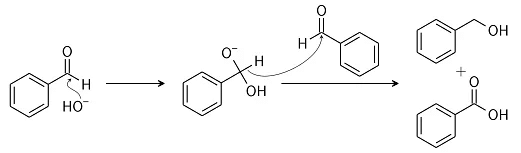
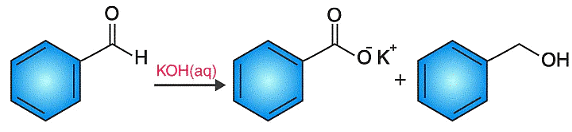
Examples of Cannizzaro reaction for better understanding: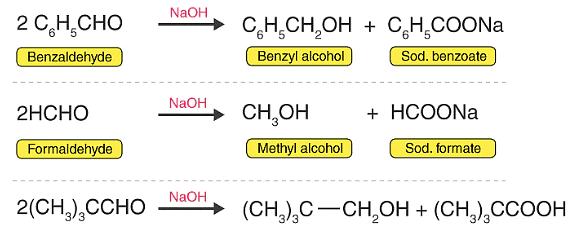
Applications of Cannizzaro Reaction:
- Formation of Carboxylic Acid and Alcohol from aldehydes devoid of α-hydrogen.
Variations of This Reaction For Further Study:
1. Crossed Cannizzaro Reaction:
- This reaction basically includes a mixture of two aldehydes having no α-hydrogen to yield all the products which are possible from the reactants used.
- The reaction between benzaldehyde and sodium formate is given below to understand the reaction easily.
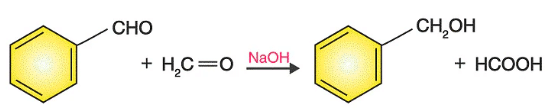
2. Intramolecular Cannizzaro Reaction:
- This reaction is within the same compound in which two aldehydic groups present which are devoid of α-hydrogen.
- The example is glyoxal having two aldehydic groups, reaction mechanism for the same is given below.