What is Clemmensen Reduction Reaction?
- Reaction used for the reduction of aldehydes or ketones to alkanes is known as Clemmensen Reduction Reaction. In a reduction reaction, there is a loss of oxygen atoms from the molecule or gain of electrons.
- Clemmensen Reduction Reaction is immensely useful for aryl-alkyl ketones reduction, formed in Friedel Crafts acylation. Acyl Benzene is formed from acylation with the help of Friedel-Crafts acylation. Clemmensen reduction reaction is used for transformation of acyl benzene to alkylbenzene and likewise, reduction of other ketones or aldehydes.
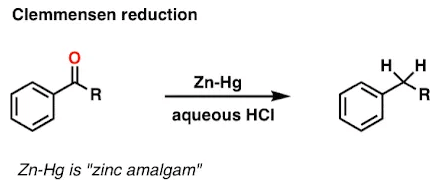
Examples:
In the given examples, when acetophenone and benzaldehyde react with a reducing agent (Zn(Hg) & HCl), they form respective hydrocarbons, i.e., ethylbenzene and methylbenzene.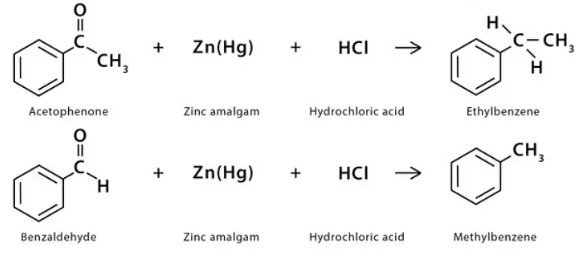
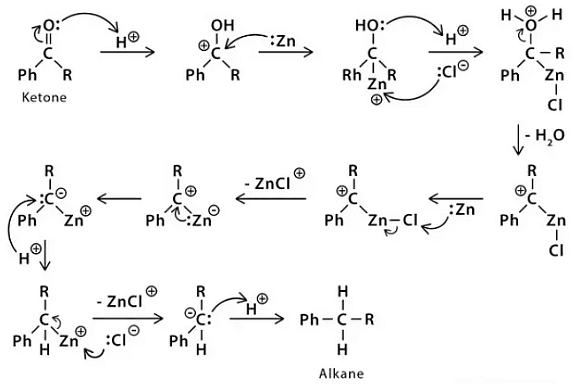
Clemmensen Reduction Reaction: Mechanism
Clemmensen reduction reaction mechanism says that when aldehydes and ketones react with zinc amalgam and concentrated hydrochloric acid, then a hydrocarbon is formed due to deoxygenation. The reduction reaction happens on the zinc surface. We still do not have a firm mechanism but two proposals are made as given below:
Carbanionic Mechanism
- Carbanionic mechanism is said to have a direct reaction on protonated carbon by zinc.
Carbenoid Mechanism
- In the carbenoid mechanism, the reactions on the metal surface of zinc reduce and take place on the surface of the zinc catalyst. This is a radical process.
- In the mechanism of Clemmensen reduction reactions, intermediacy of zinc is followed. Deoxygenationation of ketones and aldehydes takes place to form corresponding hydrocarbons. The substrate in this reaction has to be stable strong acid. The Clemmensen reduction is complementary to the Wolff-Kishner reduction, which is performed under very simple conditions.
The reaction equations given below explains Clemmensen Reduction reaction:
Wolf Kishner Reaction is similar to Clemmensen Reaction in following ways:
- Wolf Kishner Reaction involves heating of carbonyl compounds with hydrazine and potassium hydroxide to form alkanes. It is done in boiling solvents like ethylene glycol or diethylene glycol.
- Clemmensen Reaction involves reaction of carbonyl compounds and hydrazine to form hydrazones to finally form alkanes after heating.
Wolf Kishner Reaction is different to Clemmensen Reaction in following ways:
- Wolf Kishner Reaction involves conversion of carbonyl compounds to methylene compounds.
- Clemmensen Reaction involves conversion of ketones or aldehydes into alkanes.
Applications of Clemmensen Reduction
- Organic compounds can prepare alkane from alkenyl chloride (halide) which can be further create alkenyl halide
- Conversion of carbonyl group into methyl group
- Polycyclic aromatics and aromatics with unbranched side hydrocarbon chains are made.
- Reduction of aliphatic and mixed aliphatic-aromatic carbonyl compounds.
- Transformation of acyl benzene to alkyl benzene
Things to Remember
- Reaction used for the reduction of aldehydes or ketones to alkanes is known as Clemmensen Reduction Reaction.
- Clemmensen Reduction Reaction is immensely useful for aryl-alkyl ketones reduction and transformation of acyl benzene to alkyl benzene.
- There are two types of mechanisms proposed for Clemmensen Reduction Reactions- Carbanionic mechanism and Carbeniod mechanism.













