Friedel-Crafts Reaction
A Friedel-Crafts reaction is an organic coupling reaction involving an electrophilic aromatic substitution that is used for the attachment of substituents to aromatic rings. The two primary types of Friedel-Crafts reactions are the alkylation and acylation reactions. These reactions were developed in the year 1877 by the French chemist Charles Friedel and the American chemist James Crafts.
Friedel-Crafts reactions undergone by benzene is provided below.
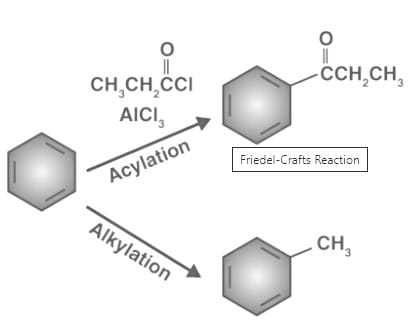
It can be noted that both these reactions involve the replacement of a hydrogen atom (initially attached to the aromatic ring) with an electrophile. Aluminium trichloride (AlCl3) is often used as a catalyst in Friedel-Crafts reactions since it acts as a Lewis acid and coordinates with the halogens, generating an electrophile in the process.
Friedel-Crafts Alkylation
Friedel-Crafts Alkylation refers to the replacement of an aromatic proton with an alkyl group. This is done through an electrophilic attack on the aromatic ring with the help of a carbocation. The Friedel-Crafts alkylation reaction is a method of generating alkylbenzenes by using alkyl halides as reactants.
The Friedel-Crafts alkylation reaction of benzene is illustrated below.
A Lewis acid catalyst such as FeCl3 or AlCl3 is employed in this reaction in order to form a carbocation by facilitating the removal of the halide. The resulting carbocation undergoes a rearrangement before proceeding with the alkylation reaction.
Mechanism
The Friedel-Crafts alkylation reaction proceeds via a three-step mechanism.
Step 1
The Lewis acid catalyst (AlCl3) undergoes reaction with the alkyl halide, resulting in the formation of an electrophilic carbocation.
Step 2
The carbocation proceeds to attack the aromatic ring, forming a cyclohexadienyl cation as an intermediate. The aromaticity of the arene is temporarily lost due to the breakage of the carbon-carbon double bond.
Step 3
The deprotonation of the intermediate leads to the reformation of the carbon-carbon double bond, restoring aromaticity to the compound. This proton goes on to form hydrochloric acid, regenerating the AlCl3 catalyst.
mechanism of the Friedel-Crafts alkylation reaction
Limitations of the Friedel-Crafts Alkylation Reaction
Some important limitations of Friedel-Crafts alkylation are listed below.
- Since the carbocations formed by aryl and vinyl halides are extremely unstable, they cannot be used in this reaction.
- The presence of a deactivating group on the aromatic ring (such as an NH2 group) can lead to the deactivation of the catalyst due to the formation of complexes.
- An excess of the aromatic compound must be used in these reactions in order to avoid polyalkylation (addition of more than one alkyl group to the aromatic compound).
- Aromatic compounds that are less reactive than mono-halobenzenes do not participate in the Friedel-Crafts alkylation reaction.
It is important to note that this reaction is prone to carbocation rearrangements, as is the case with any reaction involving carbocations.
Friedel-Crafts Acylation
The Friedel-Crafts acylation reaction involves the addition of an acyl group to an aromatic ring. Typically, this is done by employing an acid chloride (R-(C=O)-Cl) and a Lewis acid catalyst such as AlCl3. In a Friedel-Crafts acylation reaction, the aromatic ring is transformed into a ketone. The reaction between benzene and an acyl chloride under these conditions is illustrated below.

An acid anhydride can be used as an alternative to the acyl halide in Friedel-Crafts acylations. The halogen belonging to the acyl halide forms a complex with the Lewis acid, generating a highly electrophilic acylium ion, which has a general formula of RCO+ and is stabilized by resonance.
Mechanism
Friedel-Crafts acylations proceed through a four-step mechanism.
Step 1
A reaction occurs between the Lewis acid catalyst (AlCl3) and the acyl halide. A complex is formed and the acyl halide loses a halide ion, forming an acylium ion which is stabilized by resonance.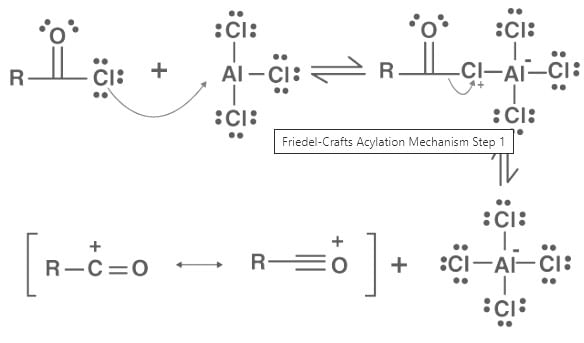
Step 2
The acylium ion (RCO+) goes on to execute an electrophilic attack on the aromatic ring. The aromaticity of the ring is temporarily lost as a complex is formed.

Step 3
The intermediate complex is now deprotonated, restoring the aromaticity to the ring. This proton attaches itself to a chloride ion (from the complexed Lewis acid), forming HCl. The AlCl3 catalyst is now regenerated.
Thus, the required acyl benzene product is obtained via the Friedel-Crafts acylation reaction.
Limitations
Despite overcoming some limitations of the related alkylation reaction (such as carbocation rearrangement and polyalkylation), the Friedel-Crafts acylation reaction has a few shortcomings.
- The acylation reaction only yields ketones. This is because formyl chloride (H(C=O)Cl) decomposes into CO and HCl when exposed to these conditions.
- The aromatic compound cannot participate in this reaction if it is less reactive than a mono-halobenzene.
- Aryl amines cannot be used in this reaction because they form highly unreactive complexes with the Lewis acid catalyst.



















