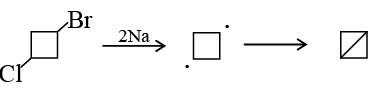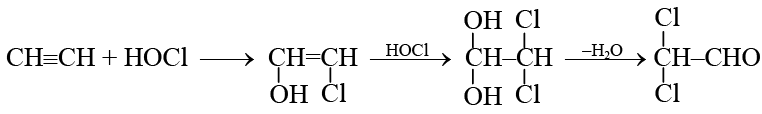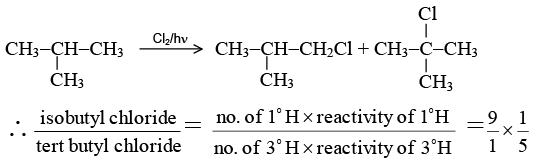JEE Advanced (Single Correct Type): Hydrocarbons | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. When acetylene is treated with HBr, the product is _______.
(a) Methyl bromide
(b) Ethylene bromide
(c) Ethyl bromide
(d) Ethylidene bromide
Correct Answer is option (d)
Ethylidene bromide is formed.
CH ≡ CH + HBr → CH2 = CHBr + HBr → CH3-CHBr2
Q.2. 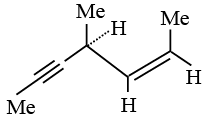 Hydrogenation of the above compound in the presence of poisoned Pd catalyst gives
Hydrogenation of the above compound in the presence of poisoned Pd catalyst gives
(a) an optically active compound.
(b) an optically inactive compound.
(c) a racemic mixture.
(d) a diastereomeric mixture.
Correct Answer is option (b)
Product contains no chiral centre.
Q.3. 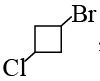 when treated with two equivalents of sodium in dry ether gives:
when treated with two equivalents of sodium in dry ether gives:
(a) 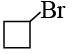
(b) 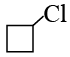
(c) 
(d) 
Correct Answer is option (d)
Q.4. Which one of the following is not an isomer of 3-Methylbut-1-yne?
(a) Pent-1-yne
(b) Buta-1,3-diene
(c) Pent-2-yne
(d) Penta-1,3-diene
Correct Answer is option (b)
Buta-1,3-diene (four carbon atoms) is not an isomer of 3-Methylbut-1-yne (five carbon atoms)
Q.5. Addition of HOCl to ethyne gives:
(a) ethyl chloride
(b) vinyl chloride
(c) dichloroacetaldehyde
(d) ethylidene chloride
Correct Answer is option (c)
Q.6. 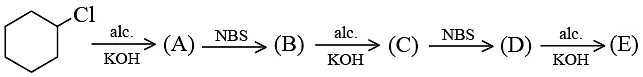 The product E is:
The product E is:
(a) 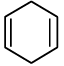
(b) 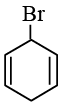
(c) 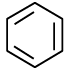
(d) 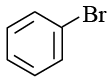
Correct Answer is option (c)
Q.7. Which of the following can be used as the halide component of a Friedel craft reaction?
(a) Chlorobenzene
(b) Bromobenzene
(c) Chloroethene
(d) Isopropyl chloride
Correct Answer is option (d)
Only isopropyl chloride can be used as the halide component for Friedel craft reaction. In all other cases, the cleavage of the C-X bond is not possible.
Q.8. Reaction of one molecule of HBr with one molecule of 1, 3-butadiene at 40ºC gives predominantly
(a) 3–bromo–1–butene under kinetically controlled conditions.
(b) 1–bromo–2–butene under thermodynamically controlled conditions.
(c) 3–bromo–1–butene under thermodynamically controlled conditions.
(d) 1–bromo–2–butene under kinetically controlled conditions.
Correct Answer is option (b)
CH2 = CH – CH = CH2 + HBr- CH = CH - CH3 [1, 4 adduct].
Thermodynamically controlled
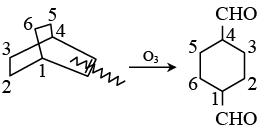
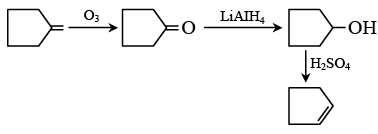
Q.9. The chemical reactions of an unsaturated compound (M) of molecular formula C8H14 are given below. Determine the possible structural formula of (M).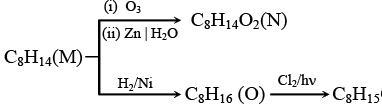 Cl(P) (only one monochloro product)
Cl(P) (only one monochloro product)
(a) 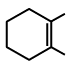
(b) 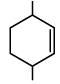
(c) 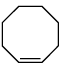
(d) 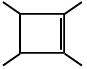
Correct Answer is option (c)
Compound (M) upon ozonolysis gives (N) which has same number of carbon as that of (M). That means it is a cyclic compound. On hydrogenation followed by chlorination it gives only one product. Hence the product is cyclooctene.
Q.10. Which of the compounds show dipole moment?
(a) 1,4-dichlorobenzene
(b) 1,2-dichlorobenzene
(c) trans-1,2-dichloroethane
(d) trans-but-2-ene
Correct Answer is option (b)
1,2-dichlorobenzene shows dipole moment because the rest are all symmetrical in nature.
Q.11. An alkene (A) 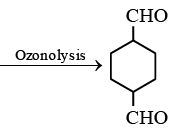 A is
A is
(a) 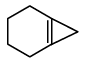
(b) 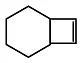
(c) 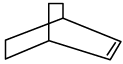
(d) 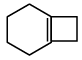
Correct Answer is option (c)
Q.12. Then which of the following is not used in; 1, 2 and 3 positions?
(a) O3/H2O
(b) LiAlH4
(c) H2SO4
(d) HI / red P
Correct Answer is option (d)
Q.13. Predict the product formed in the reaction 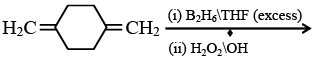 (a)
(a) 
(b) 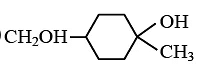
(c) 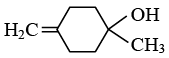
(d) 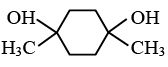
Correct Answer is option (a)
Product according to anti markonik off rule.
Q.14. Which of the following compounds will exhibit cis-trans isomerism?
(a) Butanol
(b) 2- Butyne
(c) 2-Butenol
(d) 2-Butene
Correct Answer is option (d)
CH3CH = CHCH3 will exhibit geometrical isomerism.
Q.15. Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 to form
(a) Chlorobenzene
(b) Benzyl chloride
(c) xylene
(d) toluene
Correct Answer is option (d)
Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 undergoes Friedel craft alkylation which produces toluene.
Q.16. What happens when a mixture of acetylene and hydrogen is passed over heated Lindlar’s catalyst?
(a) Ethylene and water are formed
(b) Ethane and water are formed
(c) Ethylene is formed
(d) Acetylene and ethane are formed
Correct Answer is option (c)
Ethylene is formed as a result of controlled hydrogenation of acetylene.
Q.17. The heat of hydrogenation of 1–hexene is –126 kJ mol–1. When a second double bond is introduced in the molecule, the heat of hydrogenation of the resulting compound is –230 kJ mol–1. The resulting compound (diene) is
(a) 1, 3–Hexadiene
(b) 1, 4–Hexadiene
(c) 1, 5–Hexadiene
(d) cannot predict from the given information
Correct Answer is option (a)
Since the ΔH value expected for two double bonds = -126 x 2 = -252 kJ.
While the observed is only 230 kJ
⇒ There is conjugation.
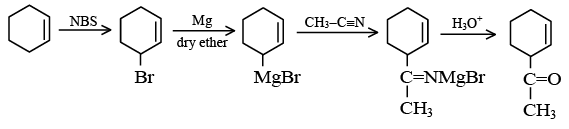
Q.18. End product of the following sequence of reaction is: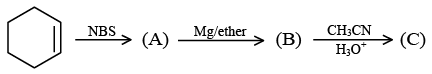 (a)
(a) 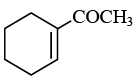
(b) 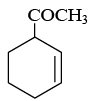
(c) 
(d) 
Correct Answer is option (b)
Q.19. When isobutane is monochlorinated in the presence of ultra-violet light, the product obtained in higher yield is
(a) n-butyl chloride
(b) isobutyl chloride
(c) sec-butyl chloride
(d) tert-butyl chloride
Correct Answer is option (b)
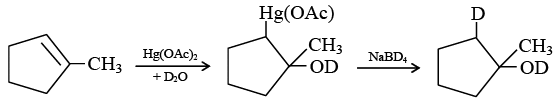
Q.20. 1-methyl cyclopentene can be converted into the following compound 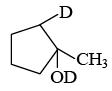 by which of these reagents?
by which of these reagents?
(a) Hg (OAc)2/D2O followed by NaBD4
(b) Hg(OAc)2/H2O followed by NaBH4
(c) Hg(OAc)2/D2O followed by NaBH4
(d) Hg(OCOCD3)2/H2O followed by NaBH4
Correct Answer is option (a)
|
481 docs|964 tests
|