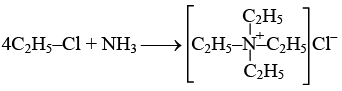JEE Advanced (Single Correct Type): Compounds Containing Nitrogen | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. What is the most basic aromatic amine’s common name?
(a) Benzenamine
(b) Benzylamine
(c) Aniline
(d) Aminobenzene
Correct Answer is option (c)
The simplest amine is aniline, which has the formula C6H5NH2. This name is also recognised by the IUPAC. The IUPAC designation for it is benzenamine, although it’s also known as aminobenzene.
Q.2. What is the correct name for a molecule that has two amino groups in opposing (para) locations around a benzene ring?
(a) Benzenediamine
(b) Benzene-1,4-diamine
(c) p-Aminoaniline
(d) 4-Aminobenzenamine
Correct Answer is option (b)
Both amino groups (in para positions to one other) are given equal weight and the primary complex is given the prefix di. The prefix is also numbered 1 and 4, indicating the places in the benzene ring where amino groups are present.
Q.3. When acetamide is converted to methylamine, what is the name of the reaction?
(a) Friedel-Craft’s reaction
(b) Hofmann reaction
(c) Hoffmann bromamide degradation reaction
(d) Hinsberg reaction
Correct Answer is option (c)
When bromine is added to an amide in an aqueous or ethanolic sodium hydroxide solution, the amide degrades, resulting in the creation of primary amine. Hoffmann bromamide degradation reaction is a reaction that involves the degradation of an amide.
Q.4. For which of the following is the Hinsberg approach used?
(a) Preparation of primary amines
(b) Separation of amine mixtures
(c) Preparation of tertiary amines
(d) Preparation of secondary amines
Correct Answer is option (b)
The Hinsberg reaction is a test for primary, secondary, and tertiary amine detection. The amine is thoroughly shaken with Hinsberg reagent in the presence of aqueous alkali in this test (either KOH or NaOH). A substrate is treated with a reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride.
Q.5. Which of the following is the IUPAC name of the chemical in which an ethyl group replaces one hydrogen of ammonia?
(a) Ethanamine
(b) Aminoethane
(c) Ethylamine
(d) Ethane amine
Correct Answer is option (a)
According to the common system and the second system, CH3CH2NH2 is known as ethylamine and aminoethane, respectively. In the IUPAC system, the amine is substituted for the ‘e’ of the alkane.
Q.6. The aromatic primary amine with the formula C7H9N has an incorrect name.
(a) Phenylaminomethane
(b) Benzylamine
(c) Benzenamine
(d) Phenylmethanamine
Correct Answer is option (c)
The phenyl group C6H5 must be present with CH4N because it is an aromatic molecule. Because it’s a primary amine, the NH2 group can be separated, leaving CH2. The compound’s formula is C6H5CH2NH2, which stands for benzylamine. C6H5NH2 is the formula for benzenamine.
Q.7. Which test can tell the difference between p-chloroaniline and anilinium hydrochloride?
a) Sandmeyer reaction
b) Carbylamine test
c) AgNO3
d) NaHCO3
Correct Answer is option (d)
An acid salt, anilinium hydrochloride, liberates CO2 from NaHCO3. However, because p-chloro aniline is basic rather than acidic, it does not release CO2. Because p-chloro aniline lacks ionic chlorine, it does not produce white precipitate when combined with AgNO3.
Q.8. By reacting with which of the following, primary amines can be separated from secondary and tertiary amines?
(a) Chloroform alone
(b) Methyl iodide
(c) Chloroform and alcoholic KOH
(d) Zinc dust
Correct Answer is option (c)
Secondary and tertiary amines do not react with CHCl3 and alc. KOH generates isocyanide, whereas primary amine does.
Q.9. Which of the following statements concerning methylamine is correct?
(a) Methylamine is stronger base than NH3
(b) Methylamine is less basic than NH3
(c) Methylamine is slightly acidic
(d) Methylamine forms salts with alkali
Correct Answer is option (a)
Due to the +I effect, the presence of an alkyl group enhances the electron density of the nitrogen atom. As a result, basic nature grows.
Q.10. Which of these substances has a lower melting point than amine?
(a) Alcohol
(b) Ether
(c) Carboxylic acid
(d) Phosphine
Correct Answer is option (d)
The characteristics of primary and secondary amines are influenced by hydrogen bonding. As a result, amines have greater melting and boiling points than the comparable phosphines but are often lower than the corresponding alcohols and carboxylic acids, ether.
Q.11. In the above process product A is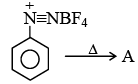
(a) Fluorobenzene
(b) Benzene
(c) 1,4-difluorobenzene
(d) 1,3-difluorobenzene
Correct Answer is option (a)
This is Baltz Schiemann reaction.
Q.12. Treatment of NH3 with excess of ethyl chloride gives:
(a) Diethylamine
(b) Ethane
(c) Tetraethylammonium chloride
(d) Methylamine
Correct Answer is option (c)
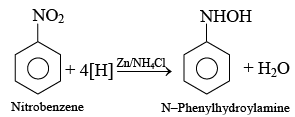
Q.13. Reduction of nitrobenzene in the presence of Zn/NH4Cl gives
(a) hydrazobenzene
(b) aniline
(c) azobenzene
(d) N-phenyl hydroxyl amine
Correct Answer is option (d)
Q.14. Which among the following amines will give carbylamine reaction?
(a) CH3–CH2–NH2
(b) CH3–NH–CH3
(c) (C6H5)3N
(d) CH3–CH2–NH–OH
Correct Answer is option (a)
Carbylamine test is responded by only 1° aliphatic/aromatic amines.
Q.15. The base with lowest pKa value is
(a) NC-CH2NH2
(b) Et3N
(c) NH3
(d) HO-CH2CH2NH2
Correct Answer is option (c)
Low value of pKa means stronger acid or weaker base. Thus as basicity decreases pKa value decreases. Due to presence of strong electron withdrawing (-I effect), NCCH2NH2 is the weakest base as it has the lowest electron density on N-atom. Hence, NCCH2CH2 has the lowest pKa value.
|
446 docs|929 tests
|