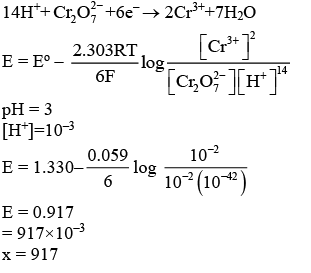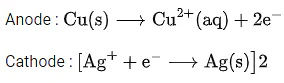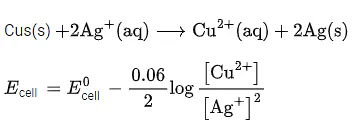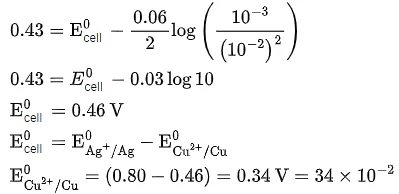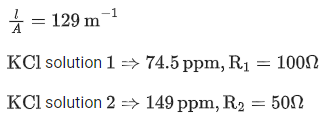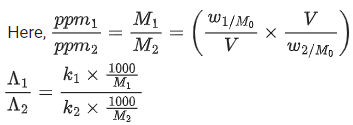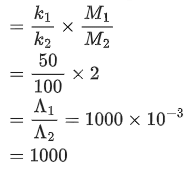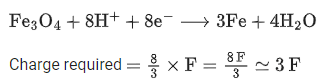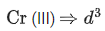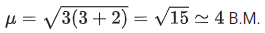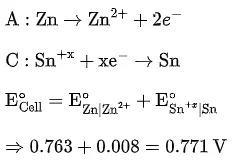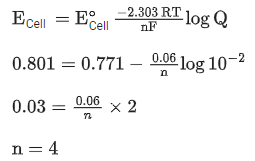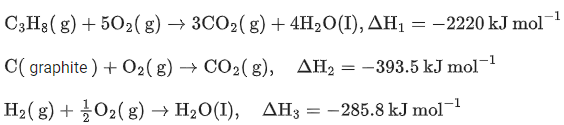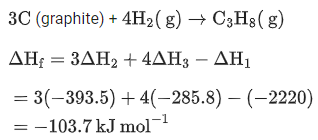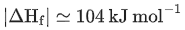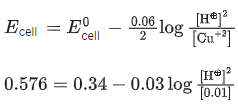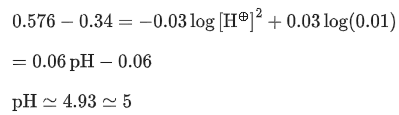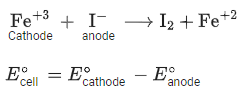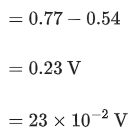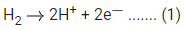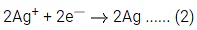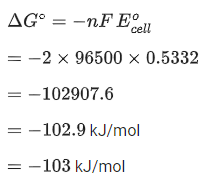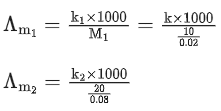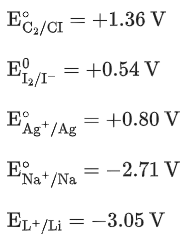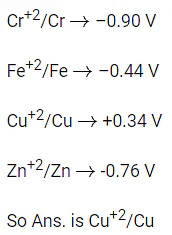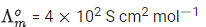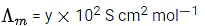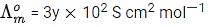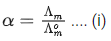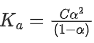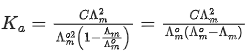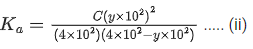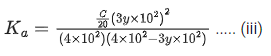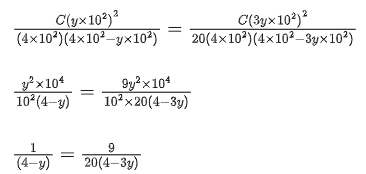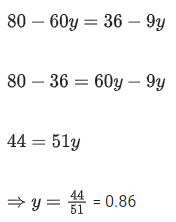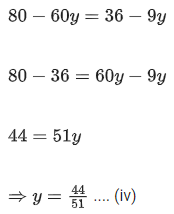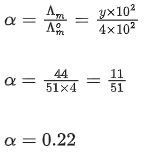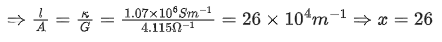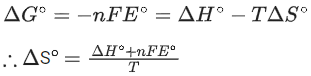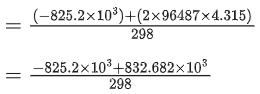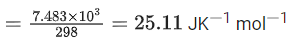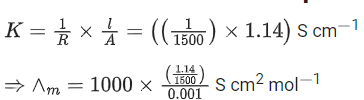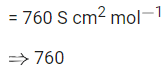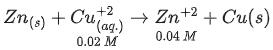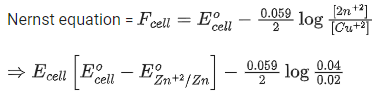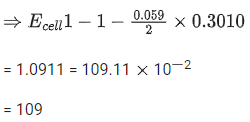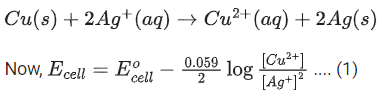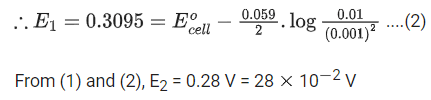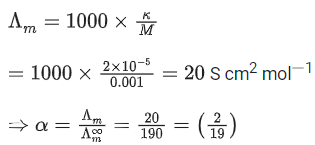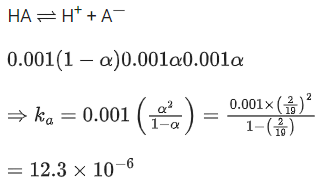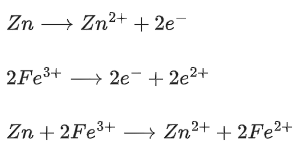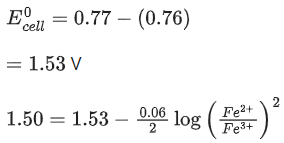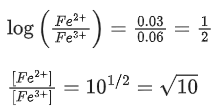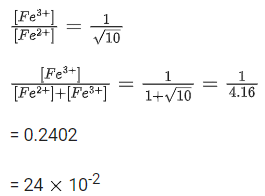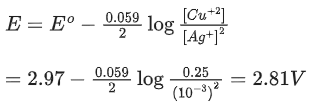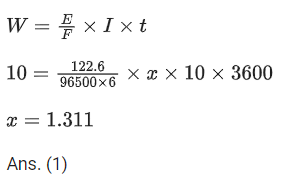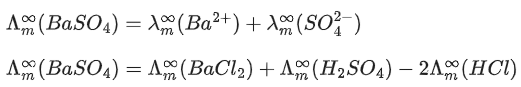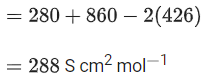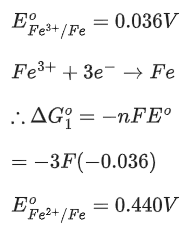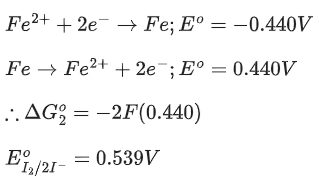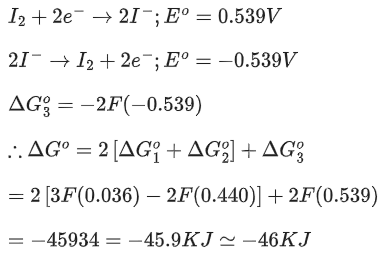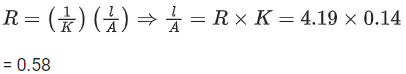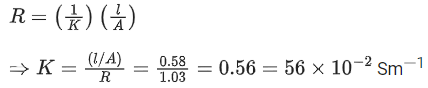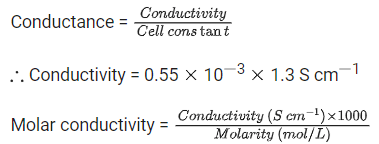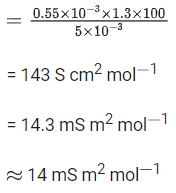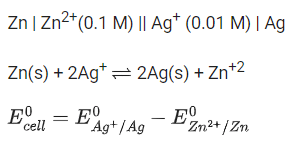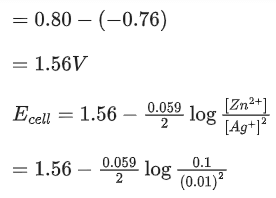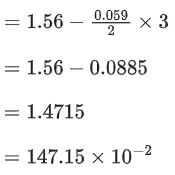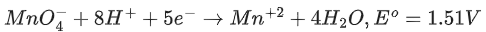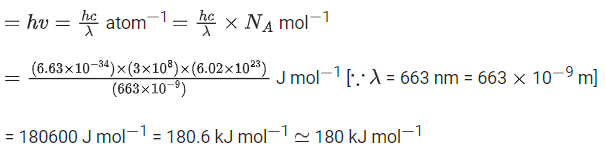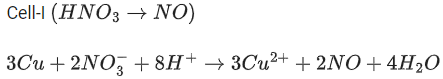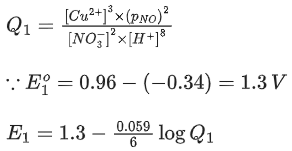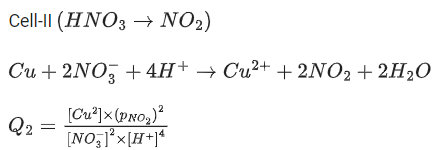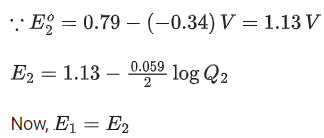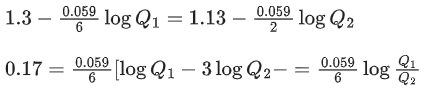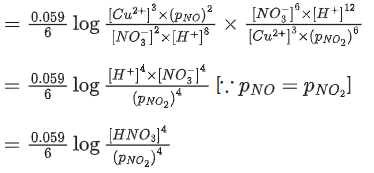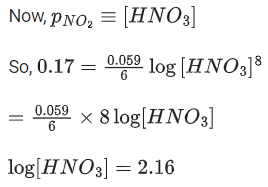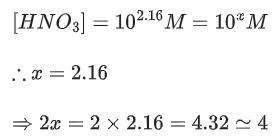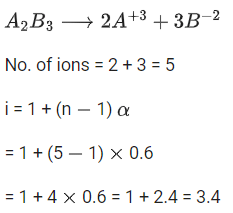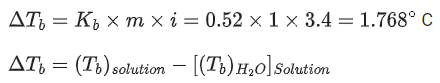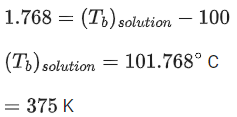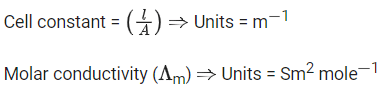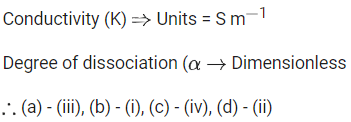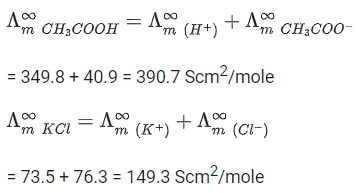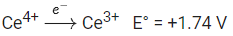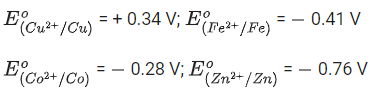JEE Previous Year Questions (2021-2025): Electrochemistry | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
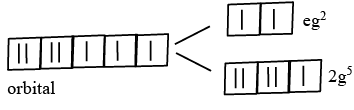
Q.1. The d-electronic configuration of [CoCl4]2− in tetrahedral crystal field is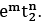 Sum of "m" and "number of unpaired electrons" is (JEE Main 2023)
Sum of "m" and "number of unpaired electrons" is (JEE Main 2023)
Ans. CO+3 → 3d7
Q.2. At 298 K, a 1 litre solution containing 10mmol of 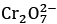 and 100mmol of Cr3+ shows a pH of 3.0.
and 100mmol of Cr3+ shows a pH of 3.0.
Given: 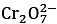 → Cr3+; E∘ = 1.330 V and
→ Cr3+; E∘ = 1.330 V and 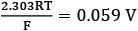 (JEE Main 2023)
(JEE Main 2023)
Ans. 917
Q.3. Consider the strong electrolytes ZmXn,UmYp and VmXn. Limiting molar conductivity ( Λ0 ) of UmYp and VmXn are 250 and 440 S cm2 mol−1, respectively. The value of (m+n+p) is
Given: (JEE Advance 2022)
λ0 is the limiting molar conductivity of ions
The plot of molar conductivity ZmXnvsc1/2 is given below.
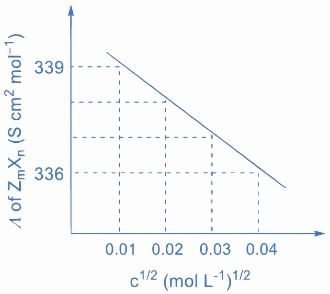
Ans. 7
Q.4. The reduction potential 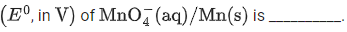 (JEE Advance 2022)
(JEE Advance 2022)
Given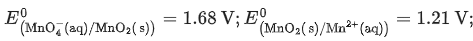
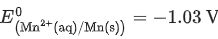
Ans. 0.74 - 0.80
Q.5. For a cell, 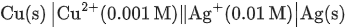 the cell potential is found to be 0.43 V at 298 K. The magnitude of standard electrode potential for Cu2+/Cu is _________ x 10-2V. (JEE Main 2022)
the cell potential is found to be 0.43 V at 298 K. The magnitude of standard electrode potential for Cu2+/Cu is _________ x 10-2V. (JEE Main 2022)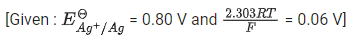
Ans. 34
Q.6. Resistance of a conductivity cell (cell constant 129 m−1) filled with 74.5ppm solution of KCl is 100Ω (labelled as solution 1). When the same cell is filled with KCl solution of 149ppm, the resistance is 50Ω (labelled as solution 2). The ratio of molar conductivity of solution 1 and solution 2 is i.e. 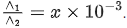 The value of x is __________. (Nearest integer)
The value of x is __________. (Nearest integer)
Given, molar mass of KCl is 74.5 g mol-1 (JEE Main 2022)
Ans. 1000
Q.7. The amount of charge in F (Faraday) required to obtain one mole of iron from Fe3O4 is ___________. (Nearest Integer) (JEE Main 2022)
Ans. 8
for Fe3O4x= +8/3
where x is oxidation state of Fe.
Q.8. The spin-only magnetic moment value of M3+ ion (in gaseous state) from the pairs Cr3+ / Cr2+, Mn3+ / Mn2+, Fe3+ / Fe2+ and Co3+ / Co2+ that has negative standard electrode potential, is ____________ B.M. [Nearest integer] (JEE Main 2022)
Ans. 4
Among the pairs given, Cr3+ / Cr2+ has negative reduction potential which is -0.41 V
Number of unpaired electrons = 3
Q.9. The cell potential for Zn|Zn2+(aq)||Snx+|Sn is 0.801 V at 298 K. The reaction quotient for the above reaction is 10−2. The number of electrons involved in the given electrochemical cell reaction is ____________. (JEE Main 2022)
(Given: 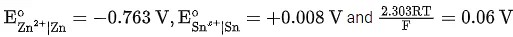 )
)
Ans. 4
From the Nernst equation,
Q.10. The enthalpy of combustion of propane, graphite and dihydrogen at 298 K are −2220.0 kJ mol−1,−393.5 kJ mol−1 and −285.8 kJ mol−1 respectively. The magnitude of enthalpy of formation of propane (C3H8) is _______________ kJmol−1. (Nearest integer) (JEE Main 2022)
Ans. 104
Enthalpy of combustion of propane, graphite and H2 at 298K are
The desired reaction is
Q.11. The cell potential for the following cell Pt |H2(g)|H+ (aq)|| Cu2+ (0.01 M)|Cu(s) is 0.576 V at 298 K. The pH of the solution is __________. (Nearest integer) (JEE Main 2022)
Given: (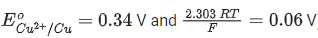 )
)
Ans. 5
Q.12. The resistance of a conductivity cell containing 0.01 M KCl solution at 298 K is 1750 Ω. If the conductivity of 0.01 M KCl solution at 298 K is 0.152 x 103 S cm-1, then the cell constant of the conductivity cell is ____________ x 10-3 cm-1. (JEE Main 2022)
Ans. 266
Molarity of KCl solution = 0.1 M
Resistance = 1750 ohm
Conductivity = 0.152x 10-3
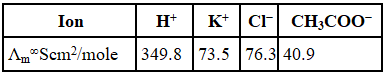
Cell constant = 0.152x 10-3 x 1750
= 266 x 10-3cm-1
Q.13. In a cell, the following reactions take place 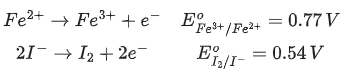
The standard electrode potential for the spontaneous reaction in the cell is x 102 V 298 K. The value of x is ____________. (Nearest Integer) (JEE Main 2022)
Ans. 23
Q.14. A solution of Fe2(SO4)3 is electrolyzed for 'x' min with a current of 1.5 A to deposit 0.3482 g of Fe. The value of x is ___________. [nearest integer] (JEE Main 2022)
Given : 1 F = 96500 C mol-1, Atomic mass of Fe = 56 g mol-1
Ans. 20
Q.15.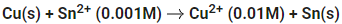
The Gibbs free energy change for the above reaction at 298 K is x 10-1 kJ mol-1. The value of x is __________. [nearest integer] (JEE Main 2022)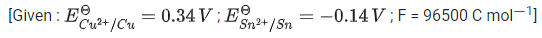
Ans. 983
Q.16. The limiting molar conductivities of NaI, NaNO3 and AgNO3 are 12.7, 12.0 and 13.3 mS m2 mol-1, respectively (all at 25°C). The limiting molar conductivity of AgI at this temperature is ____________ mS m2 mol-1. (JEE Main 2022)
Ans. 14
Q.17. For the reaction taking place in the cell :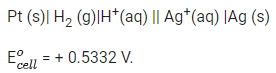
The value of ΔfG is ______________ kJ mol-1. (in nearest integer) (JEE Main 2022)
Ans. 103
# At anode, oxidation occur
# At cathode, reduction occur
Adding equation (1) and (2), we get n = 2, where n = cancelled out electron
Now,
Q.18. The quantity of electricity in Faraday needed to reduce 1 mol of 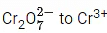 is ____________. (JEE Main 2022)
is ____________. (JEE Main 2022)
Ans. 6
Q.19. For the given reactions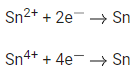
the electrode potentials are ; 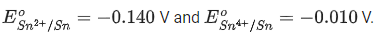 The magnitude of standard electrode potential for
The magnitude of standard electrode potential for 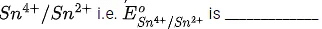 x 10-2V. (Nearest integer) (JEE Main 2022)
x 10-2V. (Nearest integer) (JEE Main 2022)
Ans. 16
Q.20. The cell potential for the given cell at 298 K
Pt| H2 (g, 1 bar) | H+ (aq) || Cu2+ (aq) | Cu(s)
is 0.31 V. The pH of the acidic solution is found to be 3, whereas the concentration of Cu2+ is 10-x M. The value of x is ___________. (JEE Main 2022)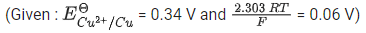
Ans. 7
Q.21. A dilute solution of sulphuric acid is electrolysed using a current of 0.10 A for 2 hours to produce hydrogen and oxygen gas. The total volume of gases produced a STP is _____________ cm3. (Nearest integer) (JEE Main 2022)
[Given : Faraday constant F = 96500 C mol-1 at STP, molar volume of an ideal gas is 22.7 L mol-1]
Ans. 127
Q.22. Match List - I with List - II. (JEE Main 2022)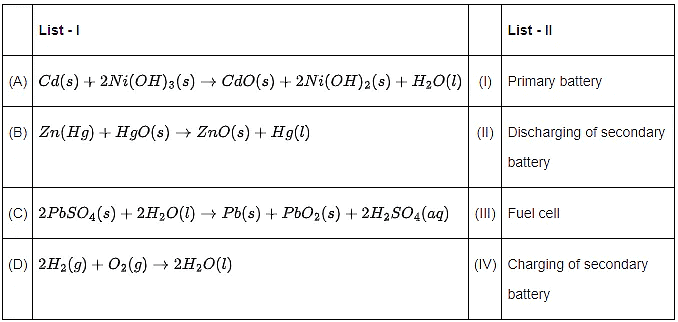 Choose the correct answer from the options given below:
Choose the correct answer from the options given below:
(a) (A)−(I),(B)−(II),(C)−(III),(D)−(IV)
(b) (A)−(IV),(B)−(I),(C)−(II),(D)−(III)
(c) (A)−(II),(B)−(I),(C)−(IV),(D)−(III)
(d) (A)−(II),(B)−(I),(C)−(III),(D)−(IV)
Ans. c
(a) Cd(s)+2Ni(OH)3( s)→CdO(s)+2Ni(OH)2( s) +H2O(l) Discharge of secondary Battery
(b) Zn(Hg)+HgO (s) →ZnO(s)+Hg(l) (Primary Battery Mercury cell)(c) 2PbSO4( s)+2H2O(l)→Pb(s)+PbO2( s)+2H2SO4(aq) (Charging of secondary Battery)
(d) 2H2( g)+O2( g)→2H2O(l) (Fuel cell)
Q.23. Given below are two statements : (JEE Main 2022)
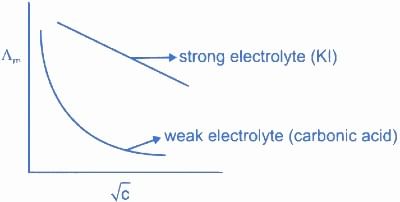
Statement I : For KI, molar conductivity increases steeply with dilution
Statement II : For carbonic acid, molar conductivity increases slowly with dilution
In the light of the above statements, choose the correct answer from the options given below:
(a) Both Statement I and Statement II are true
(b) Both Statement I and Statement II are false
(c) Statement I is true but Statement II is false
(d) Statement I is false but Statement II is true
Ans. b
For any electrolyte, molar conductivity decreases with dilution.
Both Statements are false.
Q.24. The molar conductivity of a conductivity cell filled with 10 moles of 20 mL NaCl solution is Λm1 and that of 20 moles another identical cell heaving 80 mL NaCl solution is Λm2. The conductivities exhibited by these two cells are same. The relationship between Λm2 and Λm1 is (JEE Main 2022)
(a) 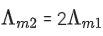
(b) 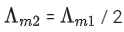
(c) 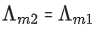
(d) 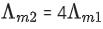
Ans. a
It is given that k1 = k2
Applying the given condition on conductivity.
Q.25. In which of the following half cells, electrochemical reaction is pH dependent? (JEE Main 2022)
(a) Pt|Fe3+,Fe2+
(b) MnO4−|Mn2+
(c) Ag|AgCl|Cl−1
(d) 1/2F2|F−
Ans. b
Reduction of MnO4− is pH dependent.
In acidic mediumMnO4−+5e− ⟶ Mn2+
In neutral medium
MnO4−+3e− ⟶ Mn4+
In basic medium
MnO4−+e ⟶ Mn6+
So, according to pH, the reaction and potential of cell changes.
Q.26. The correct order of reduction potentials of the following pairs is
A. Cl2/Cl−
B. I2/I−
C. Ag+/Ag
D. Na+/Na
E. Li+/Li
Choose the correct answer from the options given below. (JEE Main 2022)
(a) A > C > B > D > E
(b) A > B > C > D > E
(c) A > C > B > E > D
(d) A > B > C > E > D
Ans. a
Q.27. The 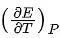 of different types of half cells are as follows:
of different types of half cells are as follows:
(Where E is the electromotive force)
Which of the above half cells would be preferred to be used as reference electrode? (JEE Main 2022)
(a) A
(b) B
(c) C
(d) D
Ans. c
A cell with less variation in EMF with temperature is preferred as a reference electrode because it can be used for a wider range of temperatures without much derivation from standard value so a cell with lessis preferred.
Q.28. In 3d series, the metal having the highest M2+/M standard electrode potential is (JEE Main 2022)
(a) Cr
(b) Fe
(c) Cu
(d) Zn
Ans. c
Q.29. At 298 K, the limiting molar conductivity of a weak monobasic acid is 4 x 102 S cm2 mol-1. At 298 K, for an aqueous solution of the acid the degree of dissociation is α and the molar conductivity is y x 102 S cm2 mol-1. At 298 K, upon 20 times dilution with water, the molar conductivity of the solution becomes 3y x 102 S cm2 mol-1.
The value of y is __________. (JEE Advance 2021)
Ans. 0.86
Degree of dissociation = α
Limiting molar conductivity,
Molar conductivity,Molar conductivity of dilution,
Concentration before dilution = C
Concentration after dilution = c/20
Using relation,
Dissociation constant,
Putting Eq. (i),
Dissociation constant before dillution,
Dissociation constant after dilution,
Comparing Eqs. (ii) and (iii),
Q.30. At 298 K, the limiting molar conductivity of a weak monobasic acid is 4 102 S cm2 mol1. At 298 K, for an aqueous solution of the acid the degree of dissociation is and the molar conductivity is y x 102 S cm2 mol-1. At 298 K, upon 20 times dilution with water, the molar conductivity of the solution becomes 3y 102 S cm2 mol-1.
The value of α is __________. (JEE Advance 2021)
Ans. 0.22
Degree of dissociation = α
Limiting molar conductivity,
Molar conductivity,Molar conductivity of dilution,
Concentration before dilution = C
Concentration after dilution = c/20
Using relation,
Dissociation constant,
Putting Eq. (i),
Dissociation constant before dillution,
Dissociation constant after dilution,
Comparing Eqs. (ii) and (iii),
Putting in Eq. (i),
The value of α is 0.22.
Q.31. If the conductivity of mercury at 0°C is 1.07 106 S m-1 and the resistance of a cell containing mercury is 0.243Ω, then the cell constant of the cell is x * 104 m-1. The value of x is ____________. (Nearest integer) (JEE Main 2021)
Ans. 26
Conductance (G) is reciprocal of resistance (R)
Relation between conductance (G),
conductivity (k) and cell constant(l/A)is given as
Q.32. Consider the following cell reaction : (JEE Main 2021)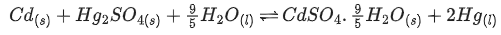
The value of 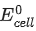 is 4.315 V at 25°C. If H = -825.2 kJ mol-1, the standard entropy change ΔS° in J K-1 is ___________. (Nearest integer) [Given : Faraday constant = 96487 C mol-1]
is 4.315 V at 25°C. If H = -825.2 kJ mol-1, the standard entropy change ΔS° in J K-1 is ___________. (Nearest integer) [Given : Faraday constant = 96487 C mol-1]
Ans. 25
Nearest integer answer is 25.
Q.33. The resistance of a conductivity cell with cell constant 1.14 cm-1, containing 0.001 M KCl at 298 K is 1500Ω . The molar conductivity of 0.001 M KCl solution at 298 K in S cm2 mol-1 is ____________. (Integer answer) (JEE Main 2021)
Ans. 760
Q.34. For the galvanic cell,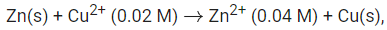
Ecell = ______________ 10-2 V. (Nearest integer) (JEE Main 2021)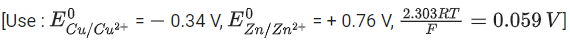
Ans. 109
Galvanic cell:
Q.35. For the cell
Cu(s) | Cu2+ (aq) (0.1 M) || Ag+(aq) (0.01 M) | Ag(s)
the cell potential E1 = 0.3095 V
For the cell
Cu(s) | Cu2+ (aq) (0.01 M) || Ag+(aq) (0.001 M) | Ag(s)
the cell potential = ____________ × 10−2 V. (Round off the nearest integer).
[Use : 2.303RT/F = 0.059] (JEE Main 2021)
Ans. 28
Cell reaction is:
Q.36. The conductivity of a weak acid HA of concentration 0.001 mol L-1 is 2.0 10-5 S cm-1. If Λ°m(HA) = 190 S cm2 mol-1, the ionization constant (Ka) of HA is equal to ______________ 10-6. (Round off to the Nearest Integer) (JEE Main 2021)
Ans. 12
Q.37. Consider the cell at 25∘C
Zn | Zn2+ (aq), (1M) || Fe3+ (aq), Fe2+ (aq) | Pt(s)
The fraction of total iron present as Fe3+ ion at the cell potential of 1.500 V is x * 10−2. The value of x is ______________. (Nearest integer) (JEE Main 2021)
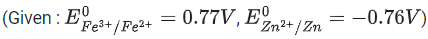
Ans. 24
Q.38. Assume a cell with the following reaction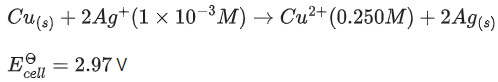
Ecell for the above reaction is ______________ V. (Nearest integer)
[Given : log 2.5 = 0.3979, T = 298 K] (JEE Main 2021)
Ans. 3
Q.39. Potassium chlorate is prepared by electrolysis of KCl in basic solution as shown by following equation.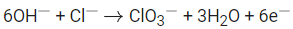
A current of xA has to be passed for 10h to produce 10.0g of potassium chlorate. The value of x is ____________. (Nearest integer) (Molar mass of KClO3 = 122.6 g mol-1, F = 96500 C) (JEE Main 2021)
Ans. 1
Q.40. The molar conductivities at infinite dilution of barium chloride, sulphuric acid and hydrochloric acid are 280, 860 and 426 S cm2 mol-1 respectively. The molar conductivity at infinite dilution of barium sulphate is _________ S cm2 mol-1. (Round off to the Nearest Integer ). (JEE Main 2021)
Ans. 288
From Kohlrausch's law
Q.41. For the reaction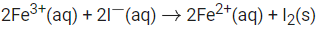 the magnitude of the standard molar free energy change, ΔrG°m = ___________ kJ (Round off to the Nearest Integer). (JEE Main 2021)
the magnitude of the standard molar free energy change, ΔrG°m = ___________ kJ (Round off to the Nearest Integer). (JEE Main 2021)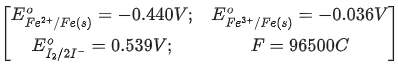
Ans. 46
Q.42. A KCl solution of conductivity 0.14 S m-1 shows a resistance of 4.19Ω in a conductivity cell. If the same cell is filled with an HCl solution, the resistance drops to 1.03Ω. The conductivity of the HCl solution is ____________ 10-2 S m-1. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 57
For KCl solution,
For HCl solution,
Q.43. A 5.0 m mol dm-3 aqueous solution of KCl has a conductance of 0.55 mS when measured in a cell of cell constant 1.3 cm-1. The molar conductivity of this solution is ___________ mSm2 mol-1. (Round off to the Nearest Integer). (JEE Main 2021)
Ans. 14
Q.44. Emf of the following cell at 298K in V is x * 10-2.
Zn|Zn2+(0.1 M)||Ag+ (0.01 M)|Ag
The value of x is _________. (Rounded off to the nearest integer) (JEE Main 2021)
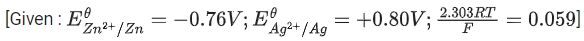
Ans. 147
Q.45. Consider the following reaction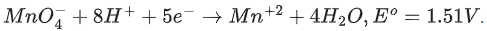
The quantity of electricity required in Faraday to reduce five moles of MnO4- is ___________. (Integer answer) (JEE Main 2021)
Ans. 25
1 mole of MnO4- required 5 moles of electrons or 5 F electricity.
∴ 5 moles of MnO4- required 25 F electricity.
Q.46. Among the following, number of metal/s which can be used as electrodes in the photoelectric cell is _________. (Integer answer) (JEE Main 2021)
(a) Li
(b) Na
(c) Rb
(d) Cs
Ans. 1
Among the given alkali metals, only cesium (Cs) is used as electrode in the photoelectric cell due to its lowest ionisation energy.
Q.47. Electromagnetic radiation of wavelength 663 nm is just sufficient to ionise the atom of metal A. The ionization enegy of metal A in kJ mol1 is __________. (Rounded off to the nearest integer)
[h = 6.63 10-34 Js, c = 3.00 x 108 ms-1, NA = 6.02 x 1023 mol-1] (JEE Main 2021)
Ans. 180
Energy of EMR = IE of the metal (A)
Q.48. Copper reduces NO3- into NO and NO2 depending upon the concentration of HNO3 in solution. (Assuming fixed [Cu2+] and  the HNO3 concentration at which the thermodynamic tendency for reduction of NO3- into NO and NO2 by copper is same is 10x M. The value of 2x is _______. (Rounded off to the nearest integer) (JEE Main 2021)
the HNO3 concentration at which the thermodynamic tendency for reduction of NO3- into NO and NO2 by copper is same is 10x M. The value of 2x is _______. (Rounded off to the nearest integer) (JEE Main 2021)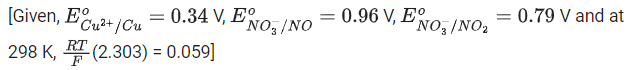
Ans. 1
Q.49. 1 molal aqueous solution of an electrolyte A2B3 is 60% ionised. The boiling point of the solution at 1 atm is _________ K. (Rounded off to the nearest integer)
[Given Kb for (H2O) = 0.52 K kg mol-1] (JEE Main 2021)
Ans. 375
Q.50. The magnitude of the change in oxidising power of the MnO4−/Mn2+ couple is x * 10−4 V, if the H+ concentration is decreased from 1M to 10−4 M at 25°C. (Assume concentration of MnO4− and Mn2+ to be same on change in H+ concentration). The value of x is ___________. [Given:2.303RT/F=0.059] (JEE Main 2021)
Ans. 3776
Reaction,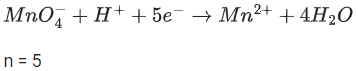
Applying Nernst equation,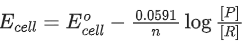
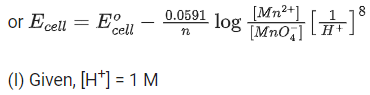
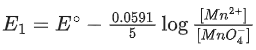
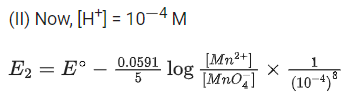
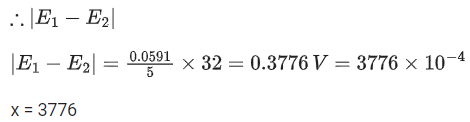
Q.51. Match List - I with List - II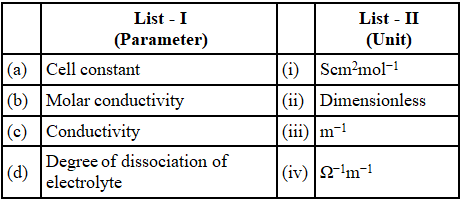
Choose the most appropriate answer from the options given below:
(a) (a)-(iii), (b)-(i), (c)-(iv), (d)-(ii)
(b) (a)-(iii), (b)-(i), (c)-(ii), (d)-(iv)
(c) (a)-(i), (b)-(iv), (c)-(iii), (d)-(ii)
(d) (a)-(ii), (b)-(i), (c)-(iii), (d)-(iv)
Ans. a
Q.52. Given below are two statements:
Statement I: The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II: Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
(a) Statement I is true but Statement II is false.
(b) Statement I is false but Statement II is true.
(c) Both Statement I and Statement II are true.
(d) Both Statement I and Statement II are false.
Ans. d
So,So, Statement I is wrong or false.
As the concentration decreases, the dilution increases which increases the degree of dissociation, thus increasing the no. of ions, which increases the molar conductance.
So Statement II is false.
Q.53. Given below are two statements :
Statement I: The Eo value for Ce4+ / Ce3+ is + 1.74 V.
Statement II: Ce is more stable in Ce4+ state than Ce3+ state.
In the light of the above statements, choose the most appropriate answer from the options given below:
(a) Statement I is incorrect but statement II is correct
(b) Both statement I and statement II are correct
(c) Both statement I and statement II are incorrect
(d) Statement I is correct but statement II is incorrect
Ans. d
Positive SRP and higher SRP means greater oxidising power. So, Ce4+ wants to reduce to Ce3+. Indicates Ce4+ is less stable than Ce3+.
Q.54. The electrode potential of M2+/M of 3d-series elements shows positive value for:
(a) Zn
(b) Fe
(c) Cu
(d) Co
Ans. c
In the electrode potential series, only copper have positive value for electrode potential because copper has lower tendency than hydrogen to form ions. So, if standard hydrogen electrode (ECell = 0) is connected to copper half-cell, the copper with be relatively less negative or less number of electrons.
∴ Electrode potential of Cushow positive value.
|
347 docs|185 tests
|