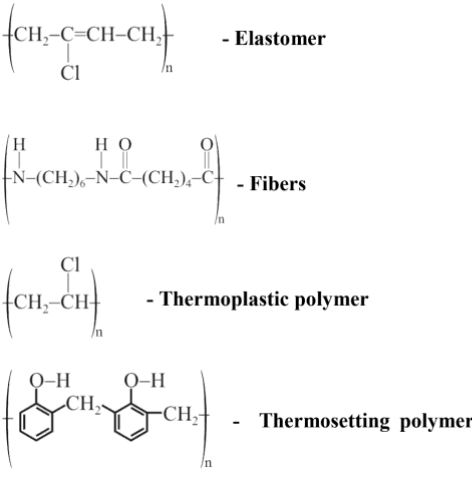
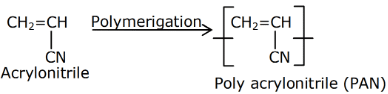
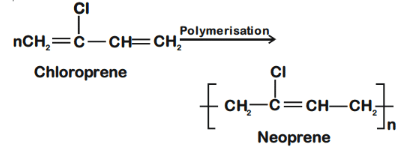
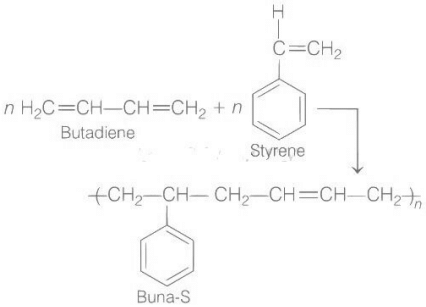
Q.1. Among the following, the correct statement(s) about polymers is(are) (JEE Advanced 2022)
(a) The polymerization of chloroprene gives natural rubber
(B) Teflon is prepared from tetrafluoroethene by heating it with persulphate catalyst at high pressures
(c) PVC are thermoplastic polymers
(d) Ethene at 350-570 K temperature and 1000-2000 atm pressure in the presence of a peroxide initiator yields high density polythene
Ans. b, c
Q.2. Which of the following is NOT a natural polymer? (JEE Main 2022)
(a) Protein
(b) Starch
(c) Rubber
(d) Rayon
Ans. d
Starch, Protein, Rubber are natural polymer.
Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products.
Q.3. Match List I with List II (JEE Main 2022) 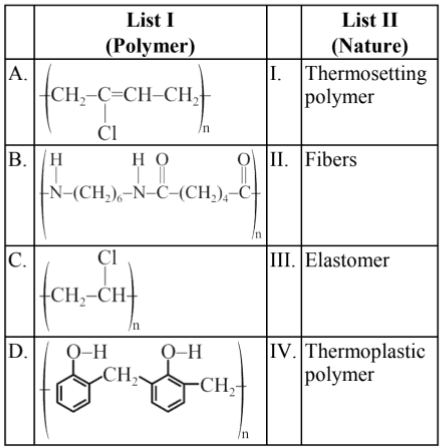
Choose the correct answer from the options given below:
(a) A-II, B-III, C-IV, D-I
(b) A-III, B-II, C-IV, D-I
(c) A-III, B-I, C-IV, D-II
(d) A-I, B-III, C-IV, D-II
Ans. b
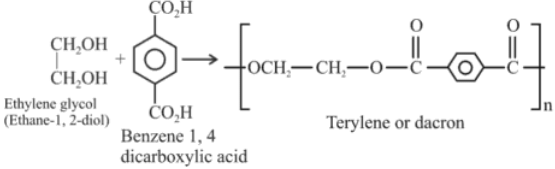
Q.4. Terylene polymer is obtained by condensation of: (JEE Main 2022)
(a) Ethane-1,2-diol and Benzene-1,3 dicarboxylic acid
(b) Propane-1, 2-diol and Benzene-1, 4 dicarboxylic acid
(c) Ethane-1,2-diol and Benzene-1, 4 dicarboxylic acid
(d) Ethane-1,2-diol and Benzene-1, 2 dicarboxylic acid
Ans. c
Q.5. Match List - I with List - II. (JEE Main 2022)
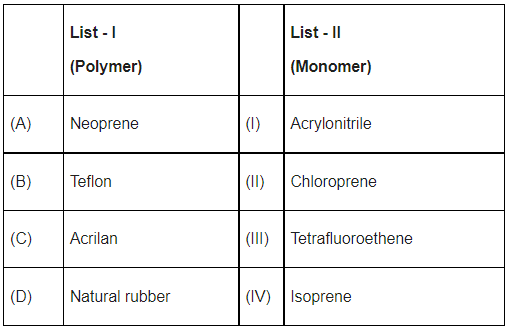
(a) (A)−(II),(B)−(III),(C)−(I),(D)−(IV)
(b) (A)−(II),(B)−(I),(C)−(III),(D)−(IV)
(c) (A)−(II),(B)−(I),(C)−(IV),(D)−(III)
(d) (A)−(I),(B)−(II),(C)−(III),(D)−(IV)
Ans. a
Q.6. Match List I with List II. (JEE Main 2022) 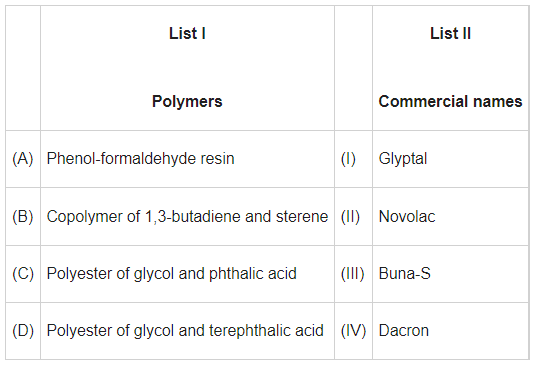
Choose the correct answer from the options given below:
(a) A-II, B-III, C-IV, D-I
(b) A-II, B-III, C-I, D-IV
(c) A-II, B-I, C-III, D-IV
(d) A-III, B-II, C-IV, D-I
Ans. b
Q.7. Vulcanization of rubber is carried out by heating a mixture of (JEE Main 2022)
(a) isoprene and styrene
(b) neoprene and sulphur
(c) isoprene and sulphur
(d) neoprene and styrene
Ans. c
When a mixture of isoprene and sulphur is heated, isoprene gets polymerised to natural rubber and then vulcanization of natural rubber with sulphur takes place.
Q.8. Match List I with List II: (JEE Main 2022) 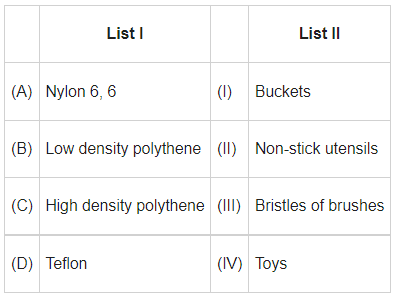
Choose the correct answer from the options given below:
(a) A-III, B-I, C-IV, D-II
(b) A-III, B-IV, C-I, D-II
(c) A-II, B-I, C-IV, D-III
(d) A-II, B-IV, C-I, D-III
Ans. b
Nylon 6,6→ used in making bristles of brushes
Low density polythene → used in making Toys
High density polythene → used in making Buckets
Teflon → used in making non-stick utensils
Q.9. Melamine polymer is formed by the condensation of: (JEE Main 2022)
(a) 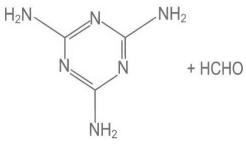
(b) 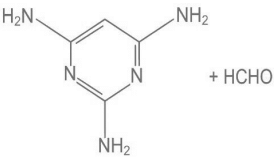
(c) 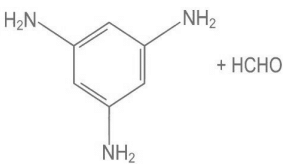
(d) 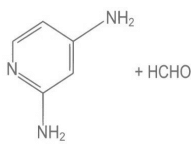
Ans. a
Q.10. Match List - I with List - II. (JEE Main 2022)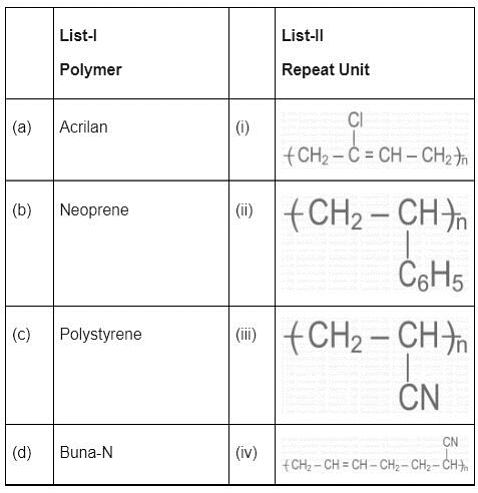
Choose the correct match from the options given below:
(a) (a)-(iii), (b)-(iv), (c)-(ii), (d)-(i)
(b) (a)-(iii), (b)-(ii), (c)-(i), (d)-(iv)
(c) (a)-(iii), (b)-(i), (c)-(ii), (d)-(iv)
(d) (a)-(ii), (b)-(iii), (c)-(i), (d)-(iv)
Ans. c
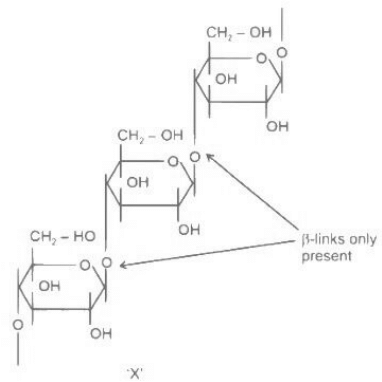
Q.11. A polysaccharide 'X' on boiling with dil H2SO4 at 393 K under 2-3 atm pressure yields 'Y'. 'Y' on treatment with bromine water gives gluconic acid. 'X' contains β-glycosidic linkages only. Compound 'X' is: (JEE Main 2022)
(a) Starch
(b) Cellulose
(c) Amylose
(d) Amylopectin
Ans. b
Cellulose contains β-glycosidic linkages only. Structure of cellulose
On boiling with dil. H2SO4 at 393 K under 2-3 atm, ' X ' forms glucose, which given gluconic acid on treatment with bromine water.
Q.12. Which of the following is an example of polyester? (JEE Main 2022)
(a) Butadiene-styrene copolymer
(b) Melamine polymer
(c) Neoprene
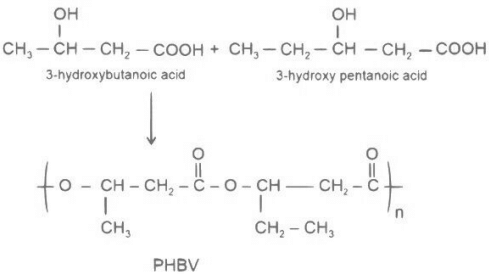
(d) Poly-β-hydroxybutyrate-co-β-hydroxyvalerate
Ans. d
Polyesters are formed by condensation reaction between alcohols and carboxylic acid. Poly- β-hydroxybutyrate-co- β-hydroxy valerate (PHBV) is a polymer obtained by condensation reaction of 3-hydroxybutanoic acid with 3-hydroxypentanoic acid.
Q.13. Which of the following is not an example of a condensation polymer? (JEE Main 2022)
(a) Nylon 6,6
(b) Decron
(c) Buna-N
(d) Silicone
Ans. c
Nylon 6,6 is a condensation polymer of hexamethylene diamine and adipic acid
Dacron is a condensation polymer of terephthalic acid and ethylene glycol.
Buna- N is an addition polymer of 1, 3-butadiene and acrylonitrile
Silicone is a condensation polymer of dialkyl silanediol.
Q.14. Which of the following sets are correct regarding polymer? (JEE Main 2022)
(A) Copolymer : Buna-S
(B) Condensation polymer : Nylon-6,6
(C) Fibres : Nylon-6,6
(D) Thermosetting polymer : Terylene
(E) Homopolymer : Buna-N
Choose the correct answer from given options below:
(a) (A), (B) and (C) are correct
(b) (B), (C) and (D) are correct
(c) (A), (C) and (E) are correct
(d) (A), (B) and (D) are correct
Ans. a
Q.15. Which is true about Buna-N? (JEE Main 2022)
(a) It is a linear polymer of 1, 3-butadiene
(b) It is obtained by copolymerization of 1, 3-butadiene and styrene
(c) It is obtained by copolymerization of 1, 3-butadiene and acrylonitrile
(d) The suffix N in Buna-N stands for its natural occurrence
Ans. c
Q.16. Which one of the following is NOT a copolymer? (JEE Main 2022)
(a) Buna-S
(b) Neoprene
(c) PHBV
(d) Butadiene-styrene
Ans. b
Q.17. Given below are two statements, one is Assertion (A) and other is Reason (R). (JEE Main 2022)
Assertion (A): Natural rubber is a linear polymer of isoprene called sis-polyisoprene with elastic properties.
Reason (R): The cis-polyisoprene molecules consist of various chains held together by strong polar interactions with coiled structure.
In the light of the above statements, choose the correct one from the options given below:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A)
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A)
(c) (A) is true but (R) is false
(d) (A) is false but (R) is true
Ans. c
Q.18. Given below are two statements : One is labelled as Assertion A and the other is labelled as Reason R. (JEE Main 2022)
Assertion A: Dacron is an example of polyester polymer.
Reason R: Dacron is made up of ethylene glycol and terephthalic acid monomers.
In the light of the above statements, choose the most appropriate answer from the options given below.
(a) Both A and R are correct and R is the correct explanation of A
(b) Both A and R are correct but R is NOT the correct explanation of A
(c) A is correct but R is not correct
(d) A is not correct but R is correct
Ans. a
Q.19. The polymer, which can be stretched and remains its original status on releasing the force is (JEE Main 2022)
(a) Bakelite
(b) Nylon 6, 6
(c) Buna-N
(d) Terylene
Ans. c
Buna – N is synthetic rubber which can be stretched and retains its original status on releasing the force.
Q.20. The Novolac polymer has mass of 963 g. The number of monomer units present in it are (JEE Main 2022)
Ans. 9
Q.21. 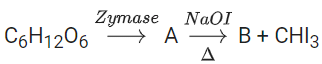
The number of carbon atoms present in the product B is _______________. (JEE Main 2022)
Ans. 1
Q.22. Monomer units of Dacron polymer are: (JEE Main 2021)
(a) Ethylene glycol and phthalic acid
(b) Ethylene glycol and terephthalic acid
(c) Glycerol and terephthalic acid
(d) Glycerol and phthalic acid
Ans. b
Q.23. Which among the following is not a polyester? (JEE Main 2021)
(a) Novolac
(b) PHBV
(c) Dacron
(d) Glyptal
Ans. a
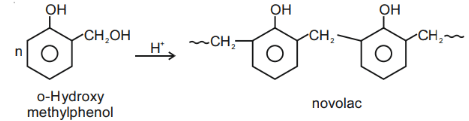
Novolac is a linear polymer of [Ph-Oh + HCHO].
So ester linkage not present.
So novolac is not a polyester.
Q.24. Monomer of Novolac is: (JEE Main 2021)
(a) 3-Hydroxybutanoic acid
(b) phenol and melamine
(c) o-Hydroxymethyl Phenol
(d) 1, 3-Butadiene and styrene
Ans. c
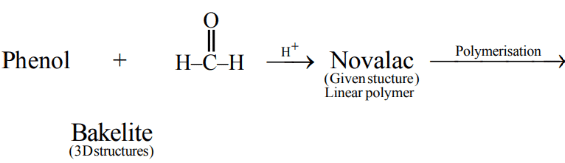
Q.25. The polymer formed on heating Novolac with formaldehyde is: (JEE Main 2021)
(a) Bakelite
(b) Polyester
(c) Melamine
(d) Nylon 6, 6
Ans. a
Novolac + formaldehyde → Bakelite
Q.26. Staggered and eclipsed conformers of ethane are: (JEE Main 2021)
(a) Polymers
(b) Rotamers
(c) Enantiomers
(d) Mirror images
Ans. b
Staggered and eclipsed conformers of ethane also known as rotamers
Q.27. A biodegradable polyamide can be made from: (JEE Main 2021)
(a) Glycine and isoprene
(b) Hexamethylene diamine and adipic acid
(c) Glycine and aminocaproic acid
(d) Styrene and caproic acid
Ans. c
A biodegradable polyamide nylon-2-Nylon-6 is made from glycine and amino caproic acid
Q.28.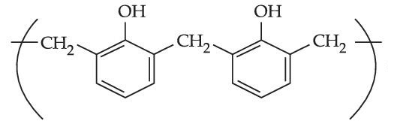
is a repeating unit for: (JEE Main 2021)
(a) Novolac
(b) Buna-N
(c) Acrilan
(d) Neoprene
Ans. a
Q.29. Match List-I with List-II: (JEE Main 2021)
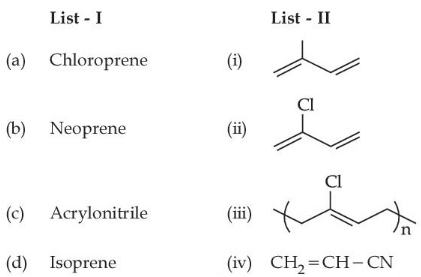
Choose the correct answer from the options given below:
(a) (a)-(iii), (b)-(iv), (c)-(ii), (d)-(i)
(b) (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
(c) (a)-(ii), (b)-(i), (c)-(iv), (d)-(iii)
(d) (a)-(iii), (b)-(i), (c)-(iv), (d)-(ii)
Ans. b
Q.30. Bakelite is a cross-linked polymer of formaldehyde and: (JEE Main 2021)
(a) PHBV
(b) Buna-S
(c) Novolac
(d) Dacron
Ans. c
Novolac (phenol formaldehyde Resin) → Bakelite
Q.31. Identify the incorrect statement from the following (JEE Main 2021)
(a) Amylose is a branched chain polymer of glucose
(b) Starch is a polymer of α-D glucose
(c) β-Glycosidic linkage makes cellulose polymer
(d) Glycogen is called as animal starch
Ans. a
Amylose is a linear chain polymer of α-D-glucose while amylopectine is branched chain polymer of α-D-glucose.
Q.32. Orlon fibres are made up of: (JEE Main 2021)
(a) Polyacrylonitrile
(b) Polyesters
(c) Polyamide
(d) Cellulose
Ans. a
Orlon fibers are made up of Polyacrylonitrile.
Q.33. Given below are two statements: (JEE Main 2021)
Statement I: Non-biodegradable wastes are generated by the thermal power plants.
Statement II: Bio-degradable detergents leads to eutrophication.
In the light of the above statements, choose the most appropriate answer from the options given below:
(a) Statement I is false but statement II is true
(b) Both statement I and statement II are true
(c) Both statement I and statement II are false
(d) Statement I is true but statement is false
Ans. b
Non-biodegradable wastes are generated by thermal power which produce fly ash.
Nowadays most of the detergents available are biodegradable. The bacteria responsible for degrading biodegradable detergent feed on it and grow rapidly. While growing, they may use up all the oxygen dissolved in water which cause Eutrophication.
Q.34. Which of the following polymer is used in the manufacture of wood laminates? (JEE Main 2021)
(a) Melamine formaldehyde resin
(b) Urea formaldehyde resin
(c) cis-poly isoprene
(d) Phenol and formaldehyde resin
Ans. b
Urea-formaldehyde resin is used in wood laminates.
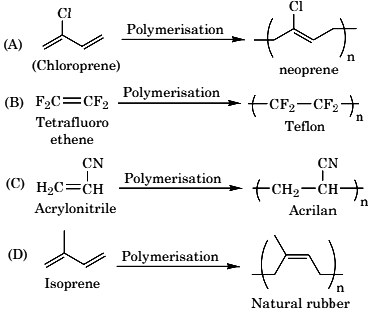
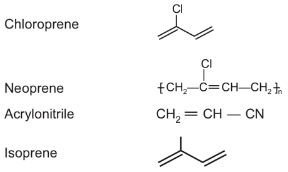
Q.35. The structure of Neoprene is: (JEE Main 2021)
(a) 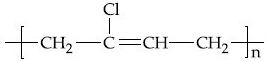
(b) 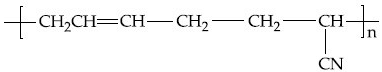
(c) 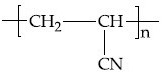
(d) 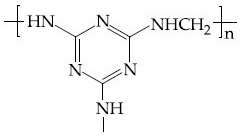
Ans. a
Neoprene is a polymer of monomer chloroprene.
Q.36. Which statement is correct? (JEE Main 2021)
(a) Buna-N is a natural polymer.
(b) Synthesis of Buna-S needs nascent oxygen.
(c) Buna-S is a synthetic and linear thermosetting polymer.
(d) Neoprene is an addition copolymer used in plastic bucket manufacturing.
Ans. b
Buna-S is an elastomer
Buna-N is a synthetic polymer
Buna-S is polymerised by addition polymerisation method which needs radical initiator for chain propagation step. Nascent oxygen can be used as an Radical initiator.
Neoprene is a synthetic rubber.
Q.37. In polymer Buna-S : 'S' stands for: (JEE Main 2021)
(a) Sulphur
(b) Styrene
(c) Strength
(d) Sulphonation
Ans. b
Buna-S is the co-polymer of but 1, 3-diene and styrene as follows.
Styrene butadiene rubber is also called buna-S, in which ‘Bu’ stands for butadiene, ‘na’ stands for sodium and ‘S’ stands for styrene.
Since, butadiene and styrene is one of the constituent monomer of given polymer.
Q.38. Match List I with List II. (JEE Main 2021)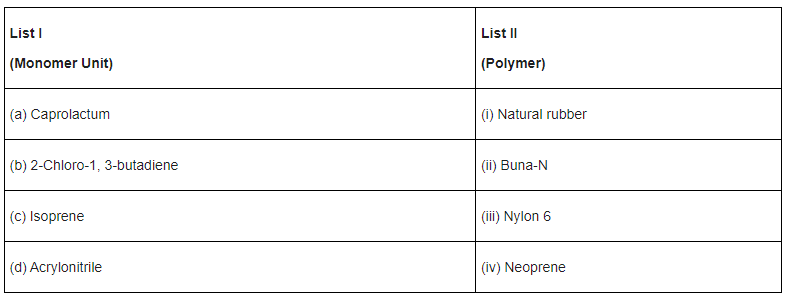
Choose the correct answer from the options given below:
(a) (a) → (iv), (b) → (iii), (c) → (ii), (d) → (i)
(b) (a) → (iii), (b) → (iv), (c) → (i), (d) → (ii)
(c) (a) → (ii), (b) → (i), (c) → (iv), (d) → (iii)
(d) (a) → (i), (b) → (ii), (c) → (iii), (d) → (iv)
Ans. b
Q.39. A peptide synthesized by the reactions of one molecule each of Glycine, Leucine, Aspartic acid and Histidine will have _________ peptide linkages. (JEE Main 2021)
Ans. 3
Combination of n amino acids gives a polypeptide with (n – 1) peptide linkages.
Similarly combination of four amino acids gives a tetrapeptide with three peptide linkages.