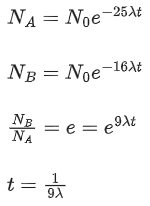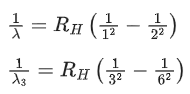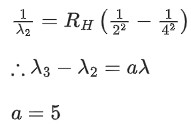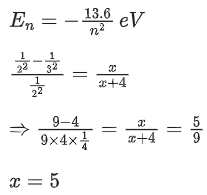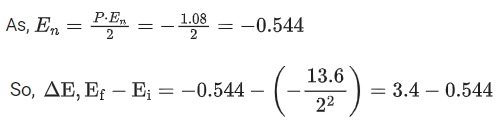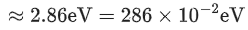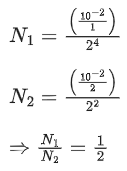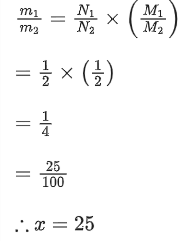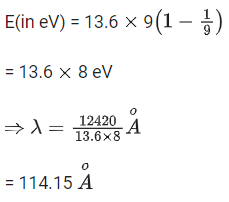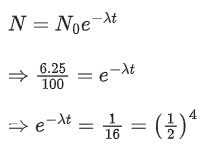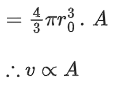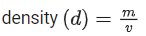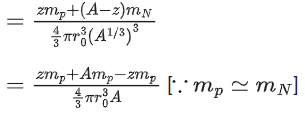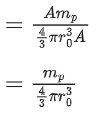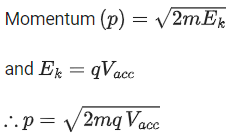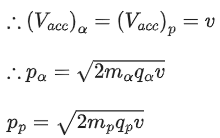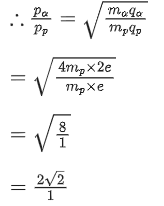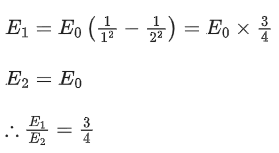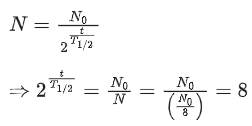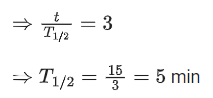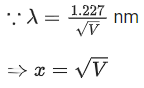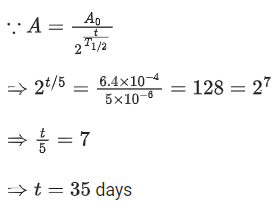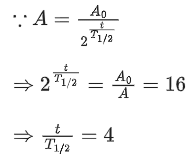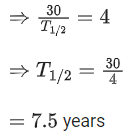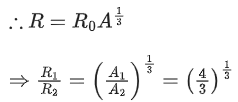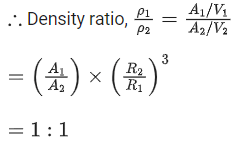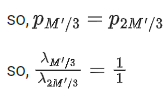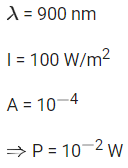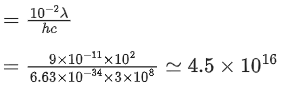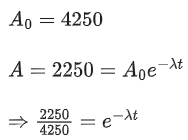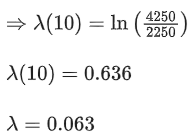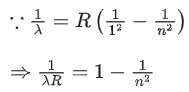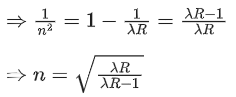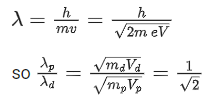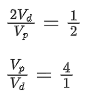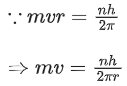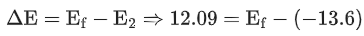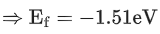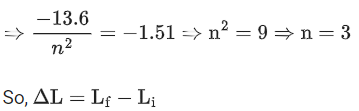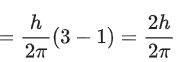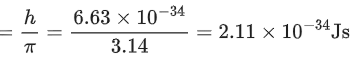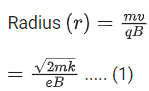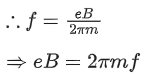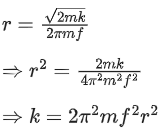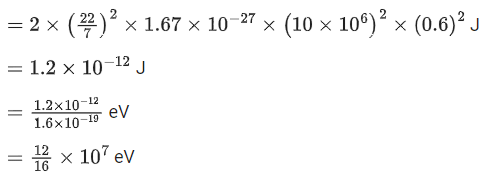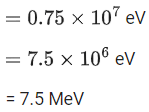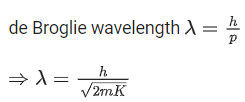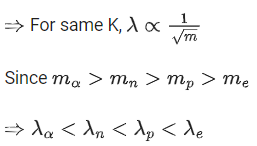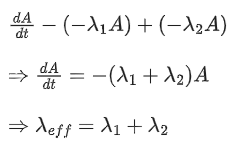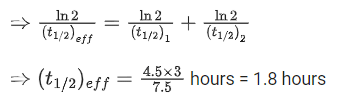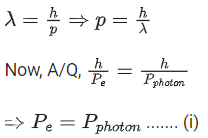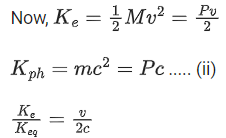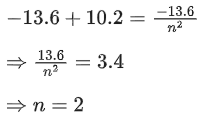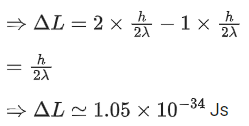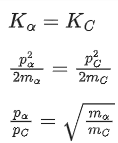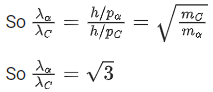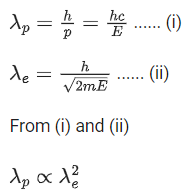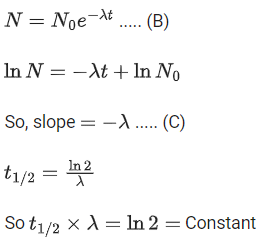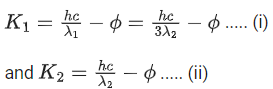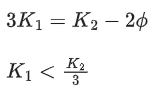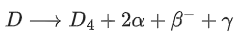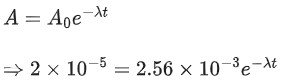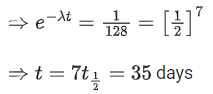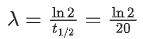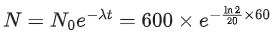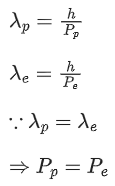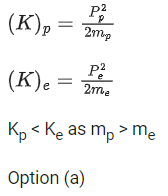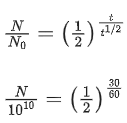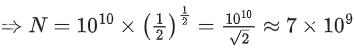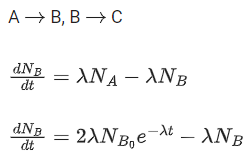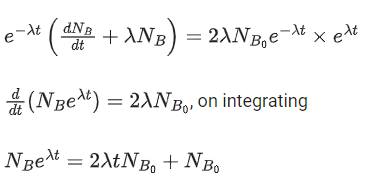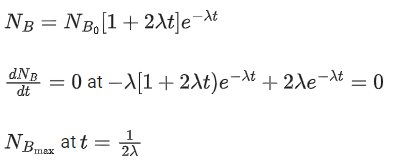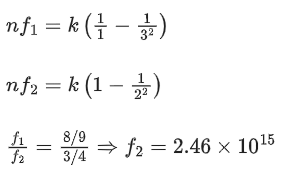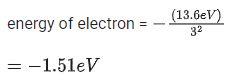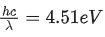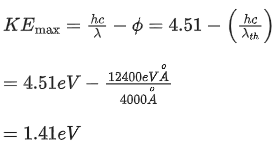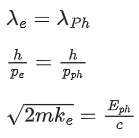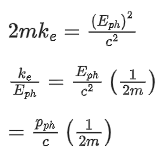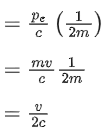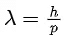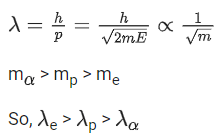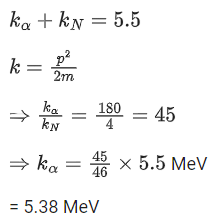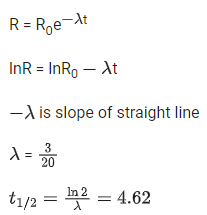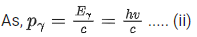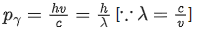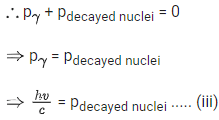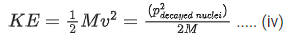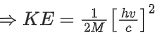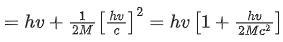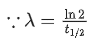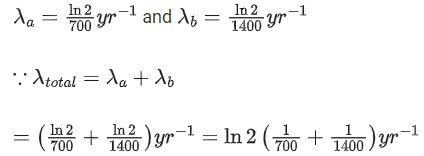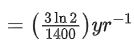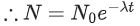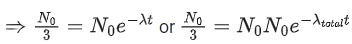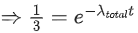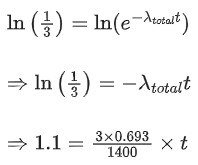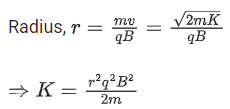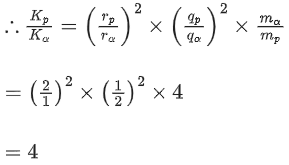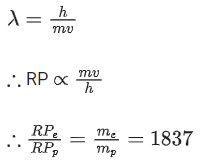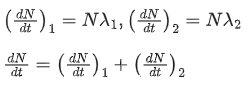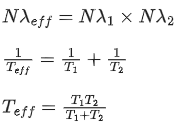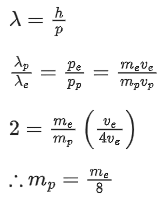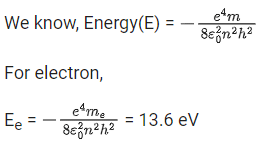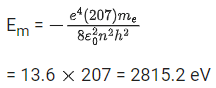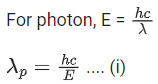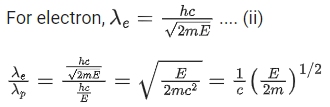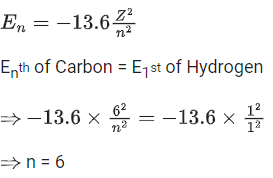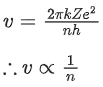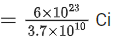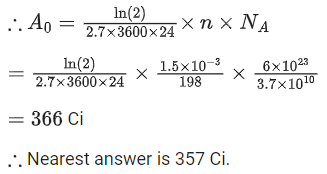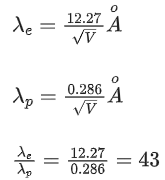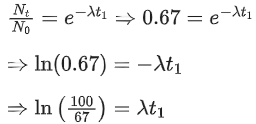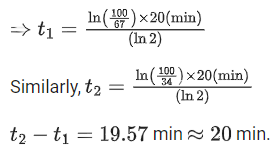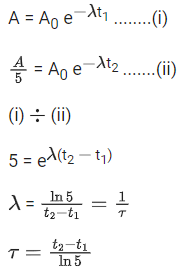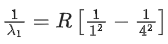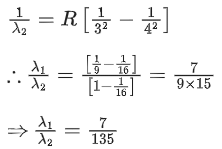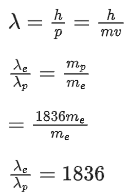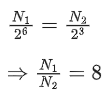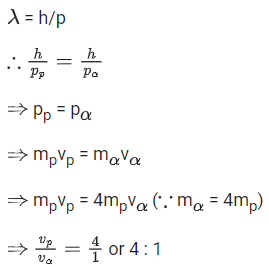Q.1. In a radioactive decay chain reaction, 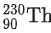 nucleus decays into
nucleus decays into 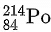
nucleus. The ratio of the number of α to β- number of particles emitted in this process is _______. (JEE Advance 2022)
Ans. 2
Q.2. The binding energy of nucleons in a nucleus can be affected by the pairwise Coulomb repulsion. Assume that all nucleons are uniformly distributed inside the nucleus. Let the binding energy of a proton be Ebp and the binding energy of a neutron be Ebn in the nucleus.
Which of the following statement(s) is(are) correct? (JEE Advance 2022)
(a) 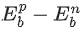 is proportional to Z(Z−1) where Z is the atomic number of the nucleus. BEbp−Ebn is proportional to A−1/3 where A is the mass number of the nucleus.
is proportional to Z(Z−1) where Z is the atomic number of the nucleus. BEbp−Ebn is proportional to A−1/3 where A is the mass number of the nucleus.
(c) 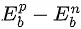 is positive.
is positive.
(d) Epb increases if the nucleus undergoes a beta decay emitting a positron.
Ans. a, b and d
Q.3. Two radioactive materials A and B have decay constants 25λ and 16λ respectively. If initially they have the same number of nuclei, then the ratio of the number of nuclei of B to that of A will be "e" after a time 1/aλ. The value of a is _________. (JEE Main 2022)
Ans. 9
Q.4. In the hydrogen spectrum, λ be the wavelength of first transition line of Lyman series. The wavelength difference will be "aλ'' between the wavelength of 3rd transition line of Paschen series and that of 2nd transition line of Balmer series where a =___________. (JEE Main 2022)
Ans. 5
Q.5. x/x+4 is the ratio of energies of photons produced due to transition of an electron of hydrogen atom from its
(i) third permitted energy level to the second level and
(ii) the highest permitted energy level to the second permitted level.
The value of x will be ____________. (JEE Main 2022)
Ans. 5
Q.6. A hydrogen atom in its first excited state absorbs a photon of energy x 10-2 eV and excited to a higher energy state where the potential energy of electron is -1.08 eV. The value of x is ______________. (JEE Main 2022)
Ans. 286
Q.7. A sample contains 10-2 kg each of two substances A and B with half lives 4 s and 8 s respectively. The ratio of their atomic weights is 1 : 2. The ratio of the amounts of A and B after 16 s is x/100. The value of x is ___________. (JEE Main 2022)
Ans. 25
∴ Mass ratio of A and B,
Q.8. A beam of monochromatic light is used to excite the electron in Li++ from the first orbit to the third orbit. The wavelength of monochromatic light is found to be x 10-10 m. The value of x is ___________. (JEE Main 2022)
[Given hc = 1242 eV nm]
Ans. 114
Q.9. The half life of a radioactive substance is 5 years. After x years a given sample of the radioactive substance gets reduced to 6.25% of its initial value. The value of x is ____________. (JEE Main 2022)
Ans. 20
Q.10. √d1 and √d2 are the impact parameters corresponding to scattering angles 60 and 90 respectively, when an α particle is approaching a gold nucleus. For d1 = x d2, the value of x will be ____________. (JEE Main 2022)
Ans. 3
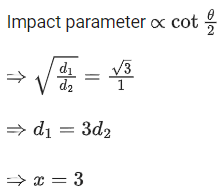
Q.11. Read the following statements : (JEE Main 2022)
(A) Volume of the nucleus is directly proportional to the mass number.
(B) Volume of the nucleus is independent of mass number.
(C) Density of the nucleus is directly proportional to the mass number.
(D) Density of the nucleus is directly proportional to the cube root of the mass number.
(E) Density of the nucleus is independent of the mass number.
Choose the correct option from the following options.
(a) (A) and (D) only.
(b) (A) and (E) only.
(c) (B) and (E) only.
(d) (A) and (C) only.
Ans. b
We know,
Radius of nucleus, r=r0A1/3
where, A = mass number
∴ Volume (v) = 4/3πr3∴ Volume is directly proportional to mass number.
We know,
∴Density of nucleus is independent of mass number.
Q.12. An α particle and a proton are accelerated from rest through the same potential difference. The ratio of linear momenta acquired by above two particles will be: (JEE Main 2022)
(a)√2:1
(b) 2√2:1
(c) 4√2:1
(d) 8:1
Ans. b
We know,Both α particle and proton are passed through same potential difference.
Q.13. Find the ratio of energies of photons produced due to transition of an electron of hydrogen atom from its (i) second permitted energy level to the first level, and (ii) the highest permitted energy level to the first permitted level. (JEE Main 2022)
(a) 3:4
(b) 4:3
(c) 1:4
(d) 4:1
Ans. a
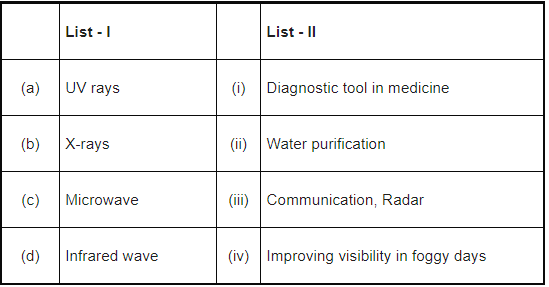
Q.14. Match List - I with List - II: (JEE Main 2022)
 Choose the correct answer from the options given below :
Choose the correct answer from the options given below :
(a) (a)-(iii), (b)-(ii), (c)-(i), (d)-(iv)
(b) (a)-(ii), (b)-(i), (c)-(iii), (d)-(iv)
(c) (a)-(ii), (b)-(iv), (c)-(iii), (d)-(i)
(d) (a)-(iii), (b)-(i), (c)-(ii), (d)-(iv)
Ans. b
UV - Water purification
X-rays - Diagnostic tool in medicine
Microwave - Communication, Radar
Infrared wave - Improving visibility in foggy days.
Q.15. A radioactive sample decays 7/8 times its original quantity in 15 minutes. The half-life of the sample is (JEE Main 2022)
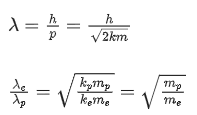
(a) 5 min
(b) 7.5 min
(c) 15 min
(d) 30 min
Ans. a
Q.16. The equation 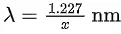 can be used to find the de-Brogli wavelength of an electron. In this equation stands for:
can be used to find the de-Brogli wavelength of an electron. In this equation stands for:
Where
m= mass of electron
P = momentum of electron
K = Kinetic energy of electron
V= Accelerating potential in volts for electron (JEE Main 2022)
(a) √mK
(b) √p
(c) √K
(d) √V
Ans. d
Q.17. The activity of a radioactive material is 6.4 x 10-4 curie. Its half life is 5 days. The activity will become 5 x 10-6 curie after: (JEE Main 2022)
(a) 7 days
(b) 15 days
(c) 25 days
(d) 35 days
Ans. d
Q.18. What is the half-life period of a radioactive material if its activity drops to
1/6th of its initial value in 30 years? (JEE Main 2022)
(a) 9.5 years
(b) 8.5 years
(c) 7.5 years
(d)10.5 years
Ans. c
Q.19. Mass numbers of two nuclei are in the ratio of 4:3. Their nuclear densities will be in the ratio of (JEE Main 2022)
(a) 4 : 3
(b) (3/4)1/3
(c) 1 : 1
(d) (4/3)1/3
Ans. c
Q.20. A nucleus of mass M at rest splits into two parts having masses M′/3 and 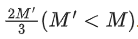 . The ratio of de Broglie wavelength of two parts will be: (JEE Main 2022)
. The ratio of de Broglie wavelength of two parts will be: (JEE Main 2022)
(a) 1:2
(b) 2:1
(c) 1:1
(d) 2:3
Ans. c
Linear momentum is conserved
Q.21. A parallel beam of light of wavelength 900 nm and intensity 100Wm−2 is incident on a surface perpendicular to the beam. The number of photons crossing 1 cm2 area perpendicular to the beam in one second is: (JEE Main 2022)
(a) 3×1016
(b) 4.5×1016
(c) 4.5×1017
(d) 4.5×1020
Ans. b
Number of photons incident per second
Q.22. The disintegration rate of a certain radioactive sample at any instant is 4250 disintegrations per minute. 10 minutes later, the rate becomes 2250 disintegrations per minute. The approximate decay constant is: (JEE Main 2022)
(Take log10 1.88 = 0.274 )
(a) 0.02min−1
(b) 2.7min−1
(c) 0.063min−1
(d) 6.3min−1
Ans. c
Q.23. Hydrogen atom from excited state comes to the ground state by emitting a photon of wavelength λ. The value of principal quantum number 'n' of the excited state will be : (R: Rydberg constant) (JEE Main 2022)
(a) 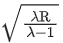
(b) 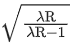
(c) 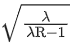
(d) 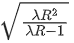
Ans. b
Q.24. The ratio of wavelengths of proton and deuteron accelerated by potential Vp and Vd is 1 : √2. Then the ratio of Vp to Vd will be: (JEE Main 2022)
(a) 1 : 1
(b) √2 : 1
(c) 2 : 1
(d) 4 : 1
Ans. d
Q.25. The momentum of an electron revolving in nth orbit is given by:
(Symbols have their usual meanings) (JEE Main 2022)
(a) 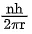
(b) 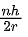
(c) 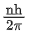
(d) 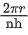
Ans. a
Q.26. A hydrogen atom in ground state absorbs 12.09 eV of energy. The orbital angular momentum of the electron is increased by: (JEE Main 2022)
(a) 1.05 × 10−34 Js
(b) 2.11 × 10−34 Js
(c) 3.16 × 10−34 Js
(d) 4.22 × 10−34 Js
Ans. b
Change in energy
Q.27. A cyclotron is working at a frequency of 10 MHz. If the radius of its dees is 60 cm. The maximum kinetic energy of accelerated proton will be:
(Take : e = 1.6 x 10-19 C, mp = 1.67 x 10-27 kg) (JEE Main 2022)
(a) 7.4 MeV
(b) 14.86 MeV
(c) 7.4 GeV
(d) 704 GeV
Ans. a
Given
f= 10x106 Hz
r = 0.6m
Charge on proton (q) = eWe know,
Also, we know,
Cyclotron oscillation frequency should be equal to the pendulum evolution frequency.
Putting this value of eB in equation (1) we get
Q.28. Choose the correct option from the following options given below: (JEE Main 2022)
(a) In the ground state of Rutherford's model electrons are in stable equilibrium. While in Thomson's model electrons always experience a net-force.
(b) An atom has a nearly continuous mass distribution in a Rutherford's model but has a highly non-uniform mass distribution in Thomson's model.
(c) A classical atom based on Rutherford's model is doomed to collapse.
(d) The positively charged part of the atom possesses most of the mass in Rutherford's model but not in Thomson's model.
Ans. c
An atom based on classical theory of Rutherford's model should collapse as the electrons in continuous circular motion that is a continuously accelerated charge should emit EM waves and so should lose energy. These electrons losing energy should soon fall into heavy nucleus collapsing the whole atom.
Q.29 In Bohr's atomic model of hydrogen, let K, P and E are the kinetic energy, potential energy and total energy of the electron respectively. Choose the correct option when the electron undergoes transitions to a higher level: (JEE Main 2022)
(a) All K, P and E increase.
(b) K decreases, P and E increase.
(c) P decreases, K and E increase.
(d) K increases, P and E decrease.
Ans. b
As electron makes transition to higher level, total energy and potential energy increases (due to negative sign) while the kinetic energy reduces.
Q.30. The ratio for the speed of the electron in the 3rd orbit of He+ to the speed of the electron in the 3rd orbit of hydrogen atom will be: (JEE Main 2022)
(a) 1:1
(b) 1:2
(c) 4:1
(d) 2:1
Ans. d
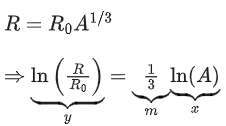
Q.31. Which of the following figure represents the variation of 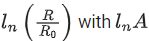 (if R = radius of a nucleus and A = its mass number) (JEE Main 2022)
(if R = radius of a nucleus and A = its mass number) (JEE Main 2022)
(a) 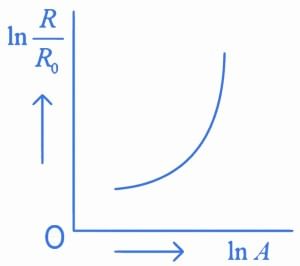
(b) 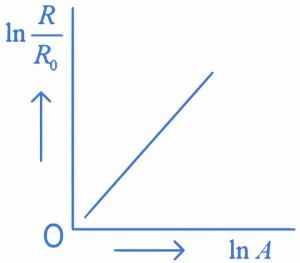
(c) 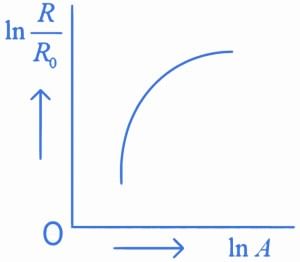
(d) 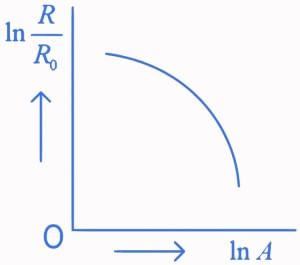
Ans. b
We know that
Straight line
Q.32. A proton, a neutron, an electron and an α particle have same energy. If λp, λn, λe and λa are the de Broglie's wavelengths of proton, neutron, electron and α particle respectively, then choose the correct relation from the following: (JEE Main 2022)
(a) λp = λn > λe > λa
(b) λa < λn < λp < λe
(c)λe < λp = λn > λa
(d) λe = λp = λn = λa
Ans. b
Where K : kinetic energy
Q.33.A radioactive nucleus can decay by two different processes. Half-life for the first process is 3.0 hours while it is 4.5 hours for the second process. The effective half-life of the nucleus will be: (JEE Main 2022)
(a) 3.75 hours
(b) 0.56 hours
(c) 0.26 hours
(d) 1.80 hours
Ans. d
Q.34. Which is the correct ascending order of wavelengths? (JEE Main 2022)
(a) λvisible < λX-ray < λgamma-ray < λmicrowave
(b) λgamma-ray< λX-ray < λvisible < λmicrowave
(c) λX-ray < λgamma-ray <λvisible < λmicrowave
(d) λmicrowave < λvisible < λgamma-ray < λX-ray
Ans. b
Wavelength of microwave is maximum then visible light then X-rays and then gamma rays so the correct order will be
λgamma-ray< λX-ray < λvisible < λmicrowave
Q.35. An electron with speed v and a photon with speed c have the same de-Broglie wavelength. If the kinetic energy and momentum of electron are Ee and pe and that of photon are Eph and pph respectively. Which of the following is correct? (JEE Main 2022)
(a) 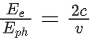
(b) 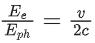
(c) 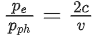
(d) 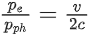
Ans. b
Q.36. A hydrogen atom in its ground state absorbs 10.2 eV of energy. The angular momentum of electron of the hydrogen atom will increase by the value of: (JEE Main 2022)
(Given, Planck's constant = 6.6 10-34 Js).
(a) 2.10 × 10−34 Js
(b) 1.05 × 10−34 Js
(c) 3.15 × 10−34 Js
(d) 4.2 × 10−34 Js
Ans. b
Q.37. An α particle and a carbon 12 atom has same kinetic energy K. The ratio of their de-Broglie wavelengths (λα:λC12) is : (JEE Main 2022)
(a) 1:√3
(b) √3:1
(c) 3:1
(d) 2 : √3
Ans. b
Q.38. Match List-I with List-II: (JEE Main 2022)
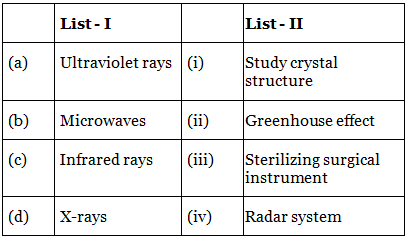
Choose the correct answer from the options given below :
(a) (a)-(iii), (b)-(iv), (c)-(ii), (d)-(i)
(b) (a)-(iii), (b)-(i), (c)-(ii), (d)-(iv)
(c) (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)
(d) (a)-(iii), (b)-(iv), (c)-(i), (d)-(ii)
Ans. a
UV rays are used to sterilize surgical material.Microwaves are used in radar system.
Infrared are used for green house effect and
X-rays are used to study crystal structure.
Q.39. The de Broglie wavelengths for an electron and a photon are λe and λp respectively. For the same kinetic energy of electron and photon, which of the following presents the correct relation between the de Broglie wavelengths of two ? (JEE Main 2022)
(a) λp∝ λe2
(b) λp∝ λe
(c) λp∝ √λe
(d) 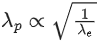
Ans. a
Q.40. Following statements related to radioactivity are given below: (JEE Main 2022)
(a) Radioactivity is a random and spontaneous process and is dependent on physical and chemical conditions.
(b) The number of un-decayed nuclei in the radioactive sample decays exponentially with time.
(c) Slope of the graph of loge (no. of undecayed nuclei) Vs. time represents the reciprocal of mean life time (τ).
(d) Product of decay constant (λ) and half-life time (T1/2) is not constant.
Choose the most appropriate answer from the options given below:
(a) (A) and (B) only
(b) (B) and (D) only
(c) (B) and (C) only
(d) (C) and (D) only
Ans. c
Radioactive decay is a random and spontaneous process it depends on unbalancing of nucleus.
Q.41. Let K1 and K2 be the maximum kinetic energies of photo-electrons emitted when two monochromatic beams of wavelength λ1 and λ2, respectively are incident on a metallic surface. If λ1 = 3λ2 then: (JEE Main 2022)
(a) 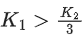
(b) 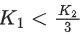
(c) 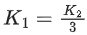
(d) 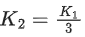
Ans. b
from (i) and (ii) we can say
Q.42. In the following nuclear reaction,
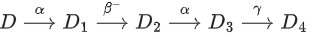
Mass number of D is 182 and atomic number is 74. Mass number and atomic number of D4 respectively will be _________. (JEE Main 2022)
(a) 174 and 71
(b) 174 and 69
(c) 172 and 69
(d) 172 and 71
Ans. a
Equivalent reaction can be written as
⇒ Mass number of D4 = Mass number of D - 2 x 4
= 182 - 8 = 174
⇒ Atomic number of D4= Atomic number of D - 2 x 2 + 1
= 74 4 + 1 = 71
Q.43. The activity of a radioactive material is 2.56 x 10-3 Ci. If the half life of the material is 5 days, after how many days the activity will become 2 x 10-5 Ci ? (JEE Main 2022)
(a) 30 days
(b) 35 days
(c) 40 days
(d) 25 days
Ans. b
Q.44. A heavy nucleus Q of half-life 20 minutes undergoes alpha-decay with probability of 60% and beta-decay with probability of 40%. Initially, the number of Q nuclei is 1000. The number of alpha-decays of Q in the first one hour is (JEE Advance 2021)
(a) 50
(b) 75
(c) 350
(d) 525
Ans. d
Out of 1000 nuclei of Q, 60% may go α-decay
600 nuclei may have α-decay
t = 1 h = 60 min
Using,
N = 7575 nuclei are left after one hour
So, number of nuclei decayed = 600 75 = 525
Q.45. A moving proton and electron have the same de-Broglie wavelength. If K and P denote the K.E. and momentum respectively. Then choose the correct option: (JEE Main 2021)
(a) Kp < Ke and Pp = Pe
(b) Kp = Ke and Pp = Pe
(c)Kp < Ke an Pp < Pe
(d) Kp > Ke and Pp = Pe
Ans. a
Q.46. A sample of a radioactive nucleus A disintegrates to another radioactive nucleus B, which in turn disintegrates to some other stable nucleus C. Plot of a graph showing the variation of number of atoms of nucleus B versus time is:
(Assume that at t = 0, there are no B atoms in the sample) (JEE Main 2021)
(a) 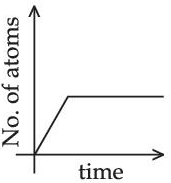
(b) 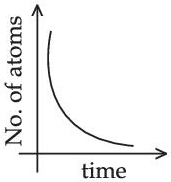
(c) 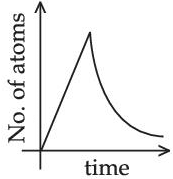
(d) 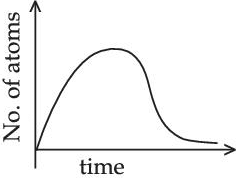
Ans. d
A → B → C (stable)
Initially no. of atoms of B = 0 at t = 0, no. of atoms of B will starts increasing & reaches maximum value when rate of decay of B = rate of formation of B.
After that maximum value, no. of atoms will starts decreasing as growth & decay both are exponential functions, so best possible graph is (d).
Q.47. There are 1010 radioactive nuclei in a given radioactive element, its half-life time is 1 minute. How many nuclei will remain after 30 seconds? (√2 = 1.414) (JEE Main 2021)
(a) 2 x 1010
(b) 7 x 109
(c) 105
(d) 4 x 1010
Ans. b
Q.48. At time t = 0, a material is composed of two radioactive atoms A and B, where NA(0) = 2NB(0). The decay constant of both kind of radioactive atoms is λ . However, A disintegrates to B and B disintegrates to C. Which of the following figures represents the evolution of NB(t) / NB(0) with respect to time t?
[NA (0) = No. of A atoms at t = 0
NB (0) = No. of B atoms at t = 0] (JEE Main 2021)
(a) 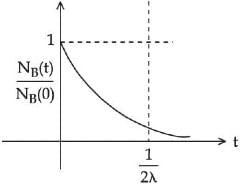
(b) 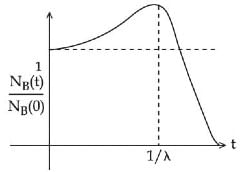
(c) 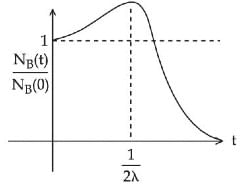
(d) 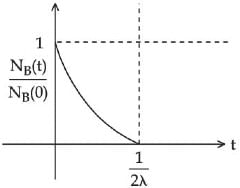
Ans. c
Q.49. A particular hydrogen like ion emits radiation of frequency 2.92 x1015 Hz when it makes transition from n = 3 to n = 1. The frequency in Hz of radiation emitted in transition from n = 2 to n = 1 will be: (JEE Main 2021)
(a) 0.44 × 1015
(b) 6.57 × 1015
(c) 4.38 × 1015
(d) 2.46 × 1015
Ans. d
Q.50. Consider the following statements: (JEE Main 2021)
A. Atoms of each element emit characteristics spectrum.
B. According to Bohr's Postulate, an electron in a hydrogen atom, revolves in a certain stationary orbit.
C. The density of nuclear matter depends on the size of the nucleus.
D. A free neutron is stable but a free proton decay is possible.
E. Radioactivity is an indication of the instability of nuclei.
Choose the correct answer from the options given below:
(a) A, B, C, D and E
(b) A, B and E only
(c) B and D only
(d) A, C and E only
Ans. b
(A) True, atom of each element emits characteristic spectrum.(B) True, according to Bohr's postulates
and hence electron resides into orbits of specific radius called stationary orbits.
(C) False, density of nucleus is constant.
(D) False, A free neutron is unstable decays into proton and electron and antineutrino.
(E) True, unstable nucleus show radioactivity.
Q.51. An electron and proton are separated by a large distance. The electron starts approaching the proton with energy 3 eV. The proton captures the electron and forms a hydrogen atom in second excited state. The resulting photon is incident on a photosensitive metal of threshold wavelength 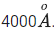 . What is the maximum kinetic energy of the emitted photoelectron? (JEE Main 2021)
. What is the maximum kinetic energy of the emitted photoelectron? (JEE Main 2021)
(a) 7.61 eV
(b) 1.41 eV
(c) 3.3 eV
(d) No photoelectron would be emitted
Ans. b
initially, energy of electron = + 3eV
finally, in 2nd excited state,
Loss in energy is emitted as photon,
So, photon energy
Now, photoelectric effect equation
Q.52. An electron moving with speed v and a photon moving with speed c, have same D-Broglie wavelength. The ratio of kinetic energy of electron to that of photon is : (JEE Main 2021)
(a) 3c/v
(b) v/3c
(c) v/2c
(d) 2c/v
Ans. c
Q.53. A particle of mass 4M at rest disintegrates into two particles of mass M and 3M respectively having non zero velocities. The ratio of de-Broglie wavelength of particle of mass M to that of mass 3M will be : (JEE Main 2021)
(a) 1:3
(b) 3:1
(c) 1:√3
(d) 1:1
Ans. d
both the particles will move with momentum same in magnitude & opposite in direction.So De-Broglie wavelength of both will be same i.e. ratio 1 : 1
Q.54. What should be the order of arrangement of de-Broglie wavelength of electron (λe), an α-particle (λa) and proton (λp) given that all have the same kinetic energy? (JEE Main 2021)
(a) λe = λp = λα
(b) λe < λp < λα
(c) λe > λp > λα
(d) λe = λp > λα
Ans. c
Q.55. An electron of mass me and a proton of mass mp are accelerated through the same potential difference. The ratio of the de-Broglie wavelength associated with the electron to that with the proton is (JEE Main 2021)
(a) 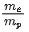
(b) 1
(c) 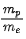
(d) 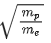
Ans. d
Q.56. A nucleus with mass number 184 initially at rest emits an α-particle. If the Q value of the reaction is 5.5 MeV, calculate the kinetic energy of the α-particle. (JEE Main 2021)
(a) 5.5 MeV
(b) 5.0 MeV
(c) 5.38 MeV
(d) 0.12 MeV
Ans. c
Q.57. For a certain radioactive process the graph between In R and t(sec) is obtained as shown in the figure. Then the value of half life for the unknown radioactive material is approximately: (JEE Main 2021)
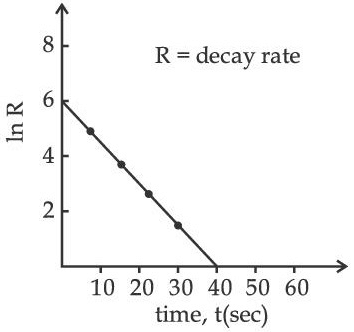 (a) 9.15 sec
(a) 9.15 sec
(b) 6.93 sec
(c) 2.62 sec
(d) 4.62 sec
Ans. d
Q.58. A nucleus of mass M emits γ -ray photon of frequency 'v'. The loss of internal energy by the nucleus is :
[Take 'c' as the speed of electromagnetic wave] (JEE Main 2021)
(a) hv
(b) 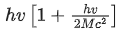
(c) 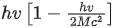
(d) 0
Ans. b
Energy of γ-ray, Eγ = hv
and momentum of γ-ray, pγ=h/λ .... (i)
From Eqs. (i) and (ii), we get
Since, during the emission of γ-ray photon, momentum is conserved.
Kinetic energy of decayed nuclei,
From Eqs. (iii) and (iv), we get
∴ Loss in internal energy = Eγ + KEdecayed nuclei
Q.59. A radioactive material decays by simultaneous emissions of two particles with half lives of 1400 years and 700 years respectively. What will be the time after which one third of the material remains ? (Take ln 3 = 1.1) (JEE Main 2021)
(a) 740 years
(b) 1110 years
(c) 700 years
(d) 340 years
Ans. a
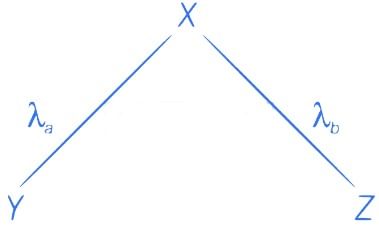
The given situation can be shown as
Here, radioactive material X is decayed into two particles Y and Z with their respective decay constant, λa and λb. It means thatwhere, t1/2(a) = 700 yr
and t1/2(b) = 1400 yr
Suppose the initial number of radioactive nuclei was N0.
where, N = number of nuclei present at time = t and N0 = number of nuclei present at time = 0
Taking log on both the sides of above equation, we get
⇒ t ≈740 yr
Q.60. A proton and an α-particle, having kinetic energies Kp and Kα respectively, enter into a magnetic field at right angles.The ratio of the radii of trajectory of proton to that of α-particle is 2 : 1. The ratio of Kp : Kα is : (JEE Main 2021)
(a) 8:1
(b) 4:1
(c) 1:8
(d) 1:4
Ans. b
Q.61. The decay of a proton to neutron is: (JEE Main 2021)
(a) always possible as it is associated only with β+ decay
(b) possible only inside the nucleus
(c) not possible as proton mass is less than the neutron mass
(d) not possible but neutron to proton conversation is possible
Ans. b
Positron emission or Beta plus decay is a subtype of radioactive decay called Beta decay, in which a proton inside a nucleus is converted into a neutron while releasing a positron and an electron neutrino.So, decay of a proton to neutron is possible only inside the nucleus. Free proton cannot decay to neutron as mass of proton is less compared to neutron so to decay into higher mass, proton need extra energy which free proton can’t get, only proton inside nucleus can get.
Q.62. The speed of electrons in a scanning electron microscope is 1 x 107 ms-1. If the protons having the same speed are used instead of electrons, then the resolving power of scanning proton microscope will be changed by a factor of : (JEE Main 2021)
(a) 1/1837
(b) 1837
(c) √1837
(d) 1/√1837
Ans. b
Resolving power (RP) ∝ 1/λ
We know, de-Broglie wavelength
Q.63. A radioactive sample disintegrates via two independent decay processes having half lives 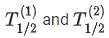 respectively. The effective half-life T1/2 of the nuclei is : (JEE Main 2021)
respectively. The effective half-life T1/2 of the nuclei is : (JEE Main 2021)
(a) 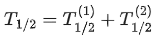
(b) 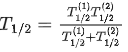
(c) 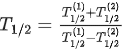
(d) None of the above
Ans. b
Q.64. A particle is travelling 4 time as fast as an electron. Assuming the ratio of de-Broglie wavelength of a particle to that of electron is 2 : 1, the mass of the particle is: (JEE Main 2021)
(a) 1/16 times the mass of e−
(b) 8 times the mass of e−
(c) 16 times the mass of e−
(d) 1/8 times the mass of e−
Ans. d
Q.65. Imagine that the electron in a hydrogen atom is replaced by a muon (μ). The mass of muon particle is 207 times that of an electron and charge is equal to the charge of an electron. The ionization potential of this hydrogen atom will be: (JEE Main 2021)
(a) 13.6 eV
(b) 28152.2 eV
(c) 331.2 eV
(d) 27.2 eV
Ans. b
mm = 207 me
In hydrogen atom one electron present. Now that the electron in hydrogen atom is replaced by a muon (μ).
For muon,
Q.66. The atomic hydrogen emits a line spectrum consisting of various series. Which series of hydrogen atomic spectra is lying in the visible region? (JEE Main 2021)
(a) Brackett series
(b) Balmer series
(c) Paschen series
(d) Lyman series
Ans. b
Balmer series of hydrogen atomic spectrum is lying in the visible region, when electron jumps from a higher energy level to n = 2 orbit.
Q.67. An electron of mass m and a photon have same energy E. The ratio of wavelength of electron to that of photon is : (c being the velocity of light) (JEE Main 2021)
(a) 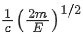
(b) 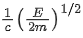
(c) 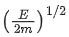
(d) 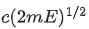
Ans. b
Q.68. Which level of the single ionized carbon has the same energy as the ground state energy of hydrogen atom? (JEE Main 2021)
(a) 8
(b) 6
(c) 1
(d) 4
Ans. b
Q.69. If an electron is moving in the nth orbit of the hydrogen atom, then its velocity (vn) for the nth orbit is given as: (JEE Main 2021)
(a) 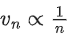
(b) 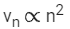
(c) 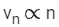
(d) 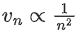
Ans. a
We know velocity of electron in nth shell of hydrogen atom is given by
Q.70. The half-life of Au198 is 2.7 days. The activity of 1.50 mg of Au198 if its atomic weight is 198 g mol-1 is, (Na = 6 x 1023/mol). (JEE Main 2021)
(a) 240 Ci
(b) 357 Ci
(c) 252 Ci
(d) 535 Ci
Ans. b
Activity, A=λN
Where, N=nNA
NA=6×1023/mol
Here, A0=λN0
We know,
Q.71. The de-Broglie wavelength associated with an electron and a proton were calculated by accelerating them through same potential of 100 V. What should nearly be the ratio of their wavelengths? (mp = 1.00727u me = 0.00055u) (JEE Main 2021)
(a) 41.4:1
(b) (1860)2:1
(c)1860:1
(d) 43:1
Ans. d
Q.72. Calculate the time interval between 33% decay and 67% decay if half-life of a substance is 20 minutes. (JEE Main 2021)
(a) 40 minutes
(b) 60 minutes
(c) 13 minutes
(d) 20 minutes
Ans. d
Q.73. A radioactive sample is undergoing α decay. At any time t1, its activity is A and another time t2, the activity is A/5, What is the average life time for the sample? (JEE Main 2021)
(a) 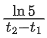
(b) 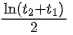
(c) 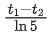
(d) 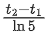
Ans. d
Let initial activity be A0
Q.74. If λ1 and λ2 are the wavelengths of the third member of Lyman and first member of the Paschen series respectively, then the value of λ1 : λ2 is: (JEE Main 2021)
(a) 7:135
(b) 7:108
(c) 1:9
(d) 1:3
Ans. a
For Lyman seriesn1 = 1, n2 = 4
For paschen series
n1 = 3, n2 = 4
Q.75. An electron of mass me and a proton of mass mp = 1836 me are moving with the same speed. The ratio of their de Broglie wavelength  will be: (JEE Main 2021)
will be: (JEE Main 2021)
(a) 1
(b) 1836
(c) 1/1836
(d) 918
Ans. b
Given mass of electron = meMass of proton = mp
given mp = 1836 me
From de-Broglie wavelength
Q.76. Two radioactive substances X and Y originally have N1 and N2 nuclei respectively. Half life of X is half of the half life of Y. After three half lives of Y, number of nuclei of both are equal. The ratio N1/N2 will be equal to: (JEE Main 2021)
(a) 1/8
(b) 3/1
(c) 1/3
(d) 8/1
Ans. d
Let Half life of x = t
then half life of y = 2t
when 3 half life of y is completed then 6 half life of x is completed.
Now x have = N1/26 nuclei
and y have = N2/23 nuclei
From question,
Q.77. The de-Broglie wavelength of a proton and α-particle are equal. The ratio of their velocities is: (JEE Main 2021)
(a) 4:2
(b) 4:3
(c) 4:1
(d) 1:4
Ans. c
Let λp, λα, mp, mα, vp, vα, pp and pα be the wavelength, mass, velocity and momentum of proton and α-particle, respectively. Given, λp = λα
As we know that
Q.78. In the given figure, the energy levels of hydrogen atom have been shown along with some transitions marked A, B, C, D and E.
The transitions A, B and C respectively represent: (JEE Main 2021)
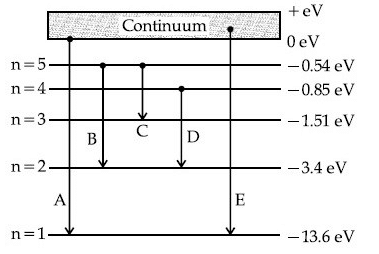 (a) The series limit of Lyman series, second member of Balmer series and second member of Paschen series.
(a) The series limit of Lyman series, second member of Balmer series and second member of Paschen series.
(b) The ionization potential of hydrogen, second member of Balmer series and third member of Paschen series.
(c) The series limit of Lyman series, third member of Balmer series and second member of Paschen series.
(d) The first member of the Lyman series, third member of Balmer series and second member of Paschen series.
Ans. c
In transition A, hydrogen atom comes from higher energy state n = to lower energy state n = 1. Hence, transition A represents series limit of Lyman series. In transition B, hydrogen atom comes from higher energy state n = 5 to lower energy state n = 2. Hence, transition B represents 3rd line of Balmer series. In transition C, hydrogen atom comes from higher energy state n = 5 to lower energy state n = 3. Hence, transition C represents 2nd line of Paschen series.
Hence, option (c) is correct.
FAQs on JEE Previous year questions (2021-22): Atom & Nuclei
| 1. What is an atom? |  |
| 2. How is the atomic number of an element determined? |  |
| 3. What is nuclear fusion? |  |
| 4. What is radioactive decay? |  |
| 5. What are isotopes? |  |