Biomolecules & Its Types | Biology Class 11 - NEET PDF Download
A cell is composed of variety of molecules (like carbon, hydrogen, oxygen) which perform various functions. Other than these basic elements, some metals and non-metals are also present as cellular materials, thence, all these materials combines in different ways in order to form various biomolecules, which are found in cells of organisms.
What are Biomolecules?
These molecules are not living, but perform various living functions. Thus, biomolecules are the organic substances (e.g., Carbohydrates, proteins, lipids, etc.) that play a major role in the structure and function of the living organism. Water is also an important and most abundant chemical compound present in the body of living organism.
How to Analyze Chemical Composition?
Units of Biomolecules Chemical Analysis & Cyanic Compounds:
To analyze the chemical composition of any organic compound in a living organism, the chemical analysis of living tissue is done. As organic compounds play a major role, it is essential to have knowledge of their molecular formula and structure.
This can be easily predicted out by performing following steps: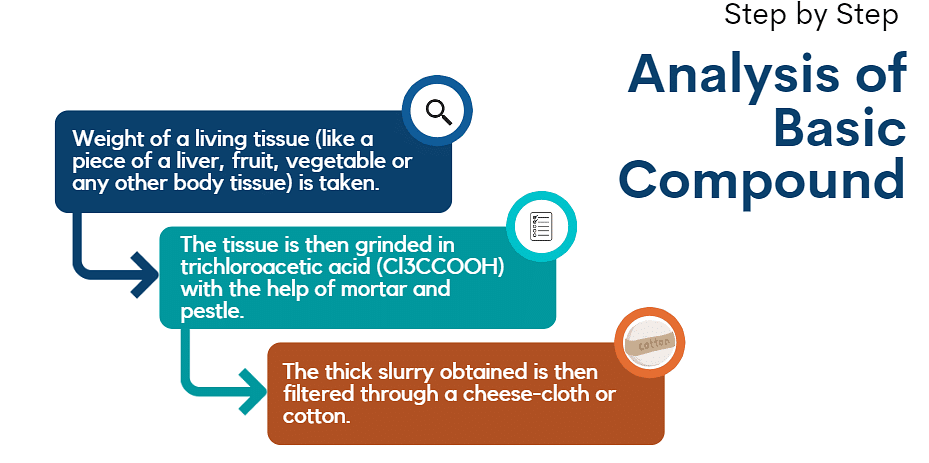
This will generate two fractions of solution:
- Filtrate or the acid-soluble fraction.
- Retentate or the acid-insoluble fraction.
Next Steps:
(iv) The extract can then be subjected to numerous separation techniques, in order to obtain the desirable compound from all other components. By successfully performing all the above steps, we can get an idea of the molecular formula and the probable structure of the compound.
(v) All the carbon compounds that we get from living tissues can be called biomolecules.
Analysis for Inorganic Compound and Elements
After the analysis of chemical composition of an organic compound in a tissue, it is necessary to do the analysis of inorganic elements and compounds. It can be done easily by performing a destructive experiment that separate inorganic compounds from organic compounds in the form of ash (contains inorganic compounds and elements). Ash is the remaining part of any living tissue which remains back after burning of all organic compounds. Ash analysis is done in order to analyze the chemical composition of different inorganic compounds and elements. It is done in the following way
(i) For analysis, a small amount of a living tissue is taken, which is oven dried till all the water evaporates.
(ii) This gives the dry weight.
(iii) After that tissue is burnt completely which results in the formation of ash. Thus, ash formed contains inorganic elements like potassium, sodium, calcium, magnesium, etc (inorganic compounds are also seen in the acid soluble fraction).
Cellular Pool
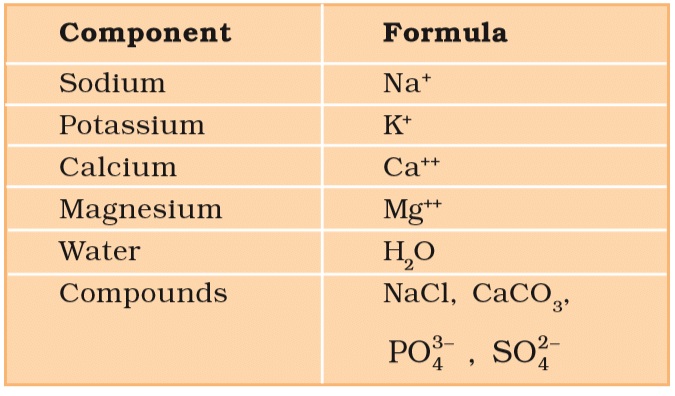
The sum total of different types of biomolecules, compounds and ions present in the cell is called cellular pool. It contains more than 5000 chemicals. List of Representative Inorganic Constituents of Living Tissues
Components Formula
Introduction of Biomacromolecules
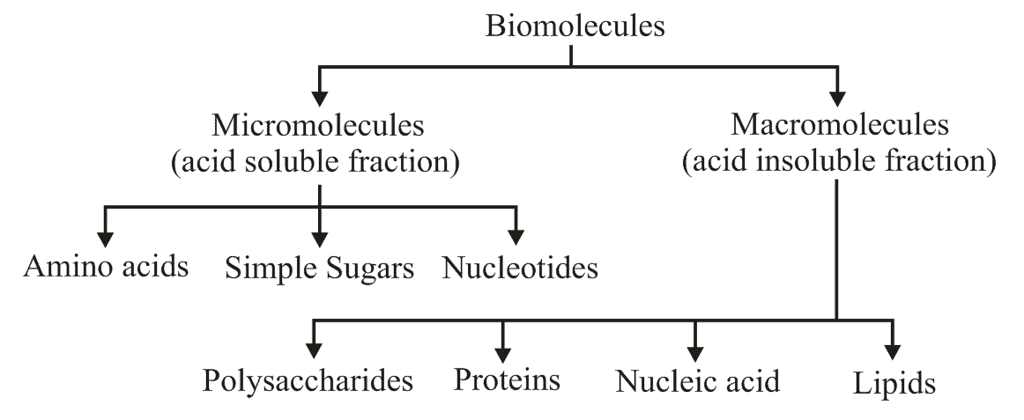
- Biomicromolecules are small in size, with low molecular weight (18-1800 Da) highly soluble (if polar) and have simple molecular conformation.
- Micromolecules can be inorganic such as water, minerals salts, gases or organic compounds such as sugars, amino acids, nucleotides, etc.
- All these compounds mentioned above are soluble fraction of filtrate except the lipids which occur as insoluble fraction of filtrate as they are mostly found in cell membrane and thereby forms vesicles, which are separated as an insoluble pool.
Macromolecules
1. Carbohydrates
These are the organic compound mainly made up of C, H and O. They are defined as polyhydroxy aldehydes and ketones. These are produced directly by the plants during photosynthesis. Carbohydrates are also known as saccharides because their major constituents are sugars. These are divided into following types:
(i) Monosaccharides: These are simplest carbohydrates which cannot be hydrolysed further into smaller components. These are generally composed of three to seven carbon atoms per molecule.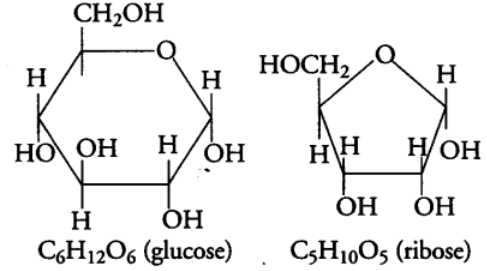
Monosaccharides are also known as reducing sugars, because they have a free aldehydic (—CHO) or ketonic (> C = O) group and can also reduce Cu2+ (cupric ions) of Benedict’s or Fehling’s solution to Cu+ (cuprous ions)., e.g., Ribose, glucose, erythrose, etc.
(ii) Oligosaccharides: These are formed by condensation of 2-6 monosaccharide molecules. The bond between two monosaccharide units is called a glycosidic bond. They are classified according to the number of their monosaccharide units or monomers as follows:
(a) Disaccharides: These are the sugars containing two monomeric units and can be further hydrolysed into smaller components. These are known as non-reducing sugars because the free aldehyde or ketone group is absent, e.g., Sucrose, maltose, lactose, etc.
(b) Trisaccharide: It contain three monomers. e.g., Raffinose.
(c) Tetrasaccharides: It contain four monomers. E.g., Stachyose and so on.
2. Amino Acids
Amino acids are organic compounds containing an amino group and an acidic group as substituent on the same carbon, i.e., the a-carbon. Hence, they are called a-amino acids. These are substituted methanes. a-carbon also bears a hydrogen and a variable group designated as R group. Thus, there are four substituent groups present on a-carbon which occupy the four different valency position. These are hydrogen, carboxyl, amino and R group. Based on the nature of R group, there are many amino acids. However, those which occur in proteins are only of twenty types.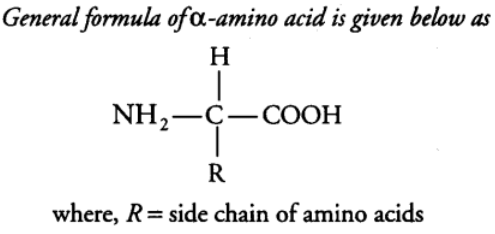
The amino group accepts a proton whereas, the carboxyl group donates a proton. So, an amino acid can act as both acid and base. Hence, it is amphoteric in nature. The R group in these proteinaceous amino acids could be a hydrogen (glycine), a methyl group (alanine), hydroxyl methyl (serine), etc.
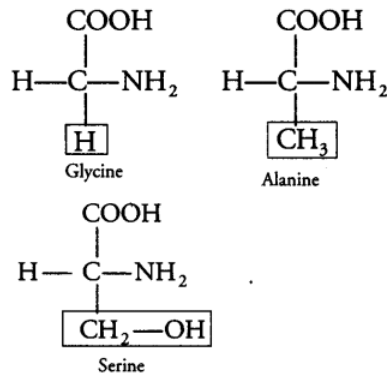
The chemical and physical properties of amino acids are essentially due to the amino, carboxyl and functional groups present.
Based on the number of amino and carboxyl group present, amino acids are categorised into following types:
(i) Acidic Amino Acids
These contain one amino group and two carboxyl group per molecule, e.g., glutamic acid and aspartic acid.
(ii) Basic Amino Acids
These contain two amino groups and one carboxyl group per molecule, e.g., Arginine, lysine and histidine.
(iii) Neutral Amino Acids
These contain one amino group and one carboxyl group per molecule, e.g., Methionine, isoleucine, serine, threonine, cysteine, glycine, alanine, valine, leucine, aspargine, glutamine and proline.
(iv) Aromatic Amino Acids
These contain aromatic rings in their side chain, e.g., Phenylalanine, tyrosine and tryptophan.
Zwitter Ion
Zwitter ion formation is anoth’er particular property of amino acid. It is a neutral molecule (with positive and negative charge), having the ionizable nature of —NH2 and —COOH groups. Hence, in splutions of different pHs, the structure of amino acid changes variably.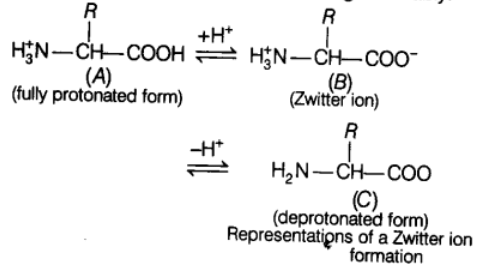
3. Lipids
Lipids are the esters of fatty acids and alcohol. These are generally insoluble in water. They could be simply fatty acids. Fatty acids are the organic acids having hydrocarbon chains that end in a carboxylic group (—COOH). The carboxylic group is attached to an R group that could be a methyl (—CH3) or ethyl (—C2H5) or higher number of —CH2 groups (1 carbon to 19 carbons), e.g., Palmitic acid has 16 carbons including carboxyl carbon.Arachidonic acid has 20 carbon atoms including the carboxyl carbon.
Depending upon the types of bonds present, fatty acids are of following two types:
(i) Saturated Fatty Acids Fatty acids which do not have double bonds, (C—C). These are generally solid at room temperature.
(ii) Unsaturated Fatty Acids Fatty acids which contain one or more than one double bonds (C=C). These are generally liquid at room temperature.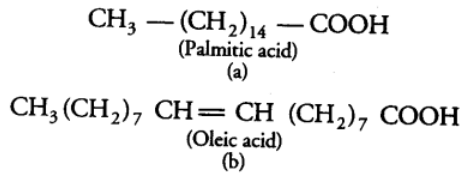
These are esters of fatty acids and various alcohol.
(i) Neutral or True Fats These are esters of fatty acids with glycerol (glycerine). They are also called glycerides. Glycerol is a simple lipid which is known as trihydroxypropane as it is an alcohol with a backbone of three carbon atoms, each carrying an —OH group. When glycerol is esterified with fatty acid it is known as triglyceride. The ester is called monoglyceride, diglyceride and triglyceride depending on the number of fatty acids attached to a glycerol.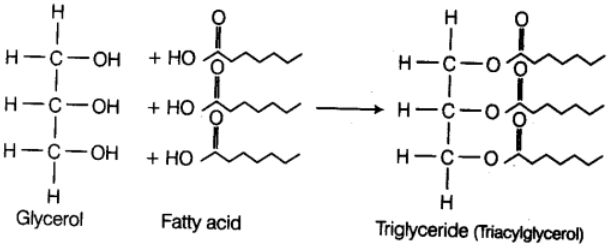
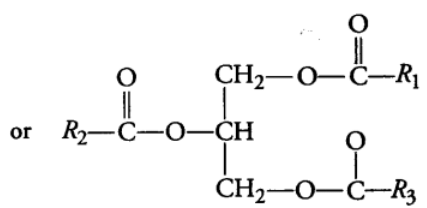
(ii) Compound or Conjugated Lipids
These are the esters of fatty acids and alcohol but contain other substances also, e.g., Phospholipids, glycolipids, cutin, suberin etc. Phospholipids are lipids which have phosphorus and phosphorylated organic compound in them. One of the common example of phospholipid is lecithin. Some tissue have complex structure of lipids, e.g., Neural tissues. (b) Oils are usually liquid at room temperature because they have low melting point, e.g., groundnut (peanut) oil, cotton seed oil, mustard oil, etc. As oils have low melting points. They remain as oils in winters also, e.g., Gingely oil.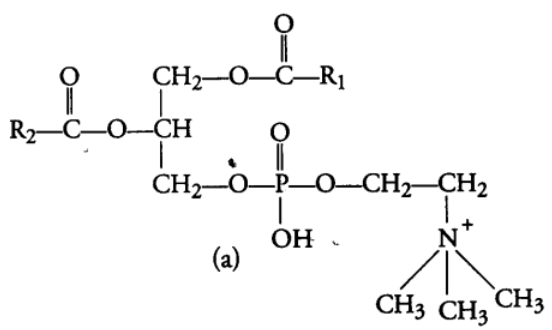
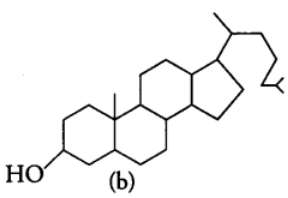
(iii) Derived Lipids
These are lipid-like substances such as sterol or derivatives of lipids, e.g., steroids, prostaglandins and terpenes. Fats are also differentiated into two main types, on the basis of their melting points at room temperature as follows (a) Hard Fats are solids at room temperature and contain long chains of fatty acids, e.g., Animal fat. Softness of butter is due to the good quantity of short chain fatty acid it contains.
4. Nucleotides
These are the monomers of nucleic acids.The nucleotides are made up of three molecules, i.e., a pentose sugar, a cyclic nitrogenous base and a phosphoric acid (phosphate group), e.g., Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid.It occurs in pentagon or fiiranose form with four carbon and one 02 forming a ring. It is present in the form of ribose or deoxyribose sugar in RNA and DNA respectively.
Nitrogenous Bases
These are the flat heterocyclic compounds having nitrogen and carbon in ring structure.
These are of basically two types:
(i) Purines: It is larger and composed of two rings. They are further of two types, i.e., Adenine (A) and Guanine (G).
(ii) Pyrimidines: It is smaller and composed of single ring. They are of further three types, i.e., Cytosine (C), Thymine (T) and Uracil (U).
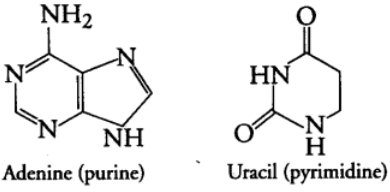
Phosphoric Acid (Phosphate Group)
It is composed of phosphoric acid. A nucleotide may have 1, 2 or 3 phosphate groups. It gives acidic nature to the nucleotide.Nucleoside
If a pentose sugar is attached to a nitrogen base by a glycosidic bond, it is called nucleoside. e.g., adenine + ribose → adenosine. Likewise guanosine, thymidine, uridine and cytidine are the examples of nucleoside. The nucleoside combines with a phosphate group at 5-position by an ester bond to form a nucleotide.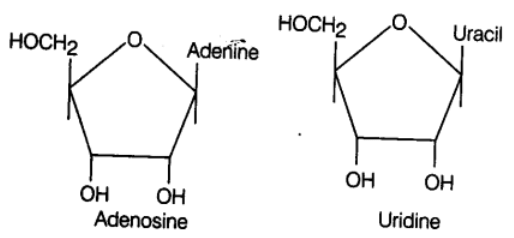
Differences between Nucleoside and Nucleotide
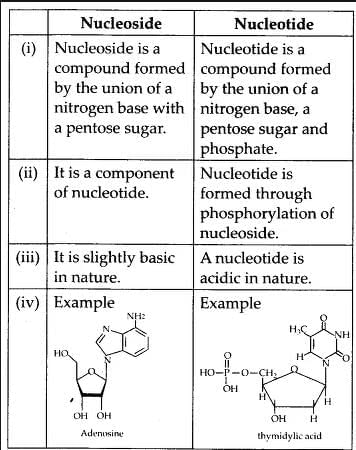
|
179 videos|531 docs|136 tests
|
FAQs on Biomolecules & Its Types - Biology Class 11 - NEET
| 1. What are biomolecules and why are they important? |  |
| 2. How can we analyze the chemical composition of biomolecules? |  |
| 3. What are the main types of biomacromolecules? |  |
| 4. How do biomolecules interact with one another in biological systems? |  |
| 5. What role do biomolecules play in metabolism? |  |

















