Biological Molecules: Carbohydrates | Biology for Grade 12 PDF Download
Biological Molecules: Reactions
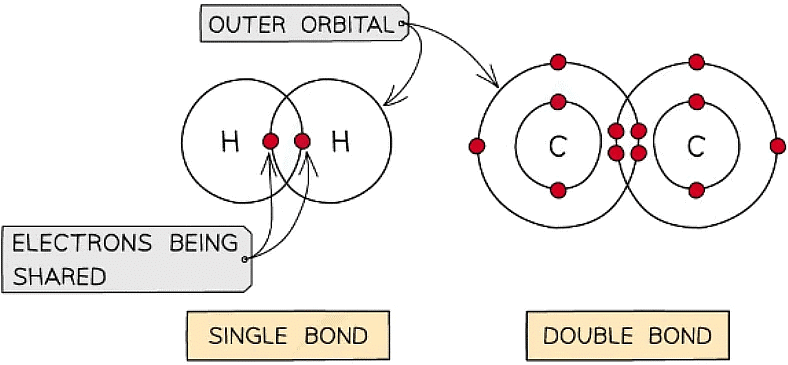
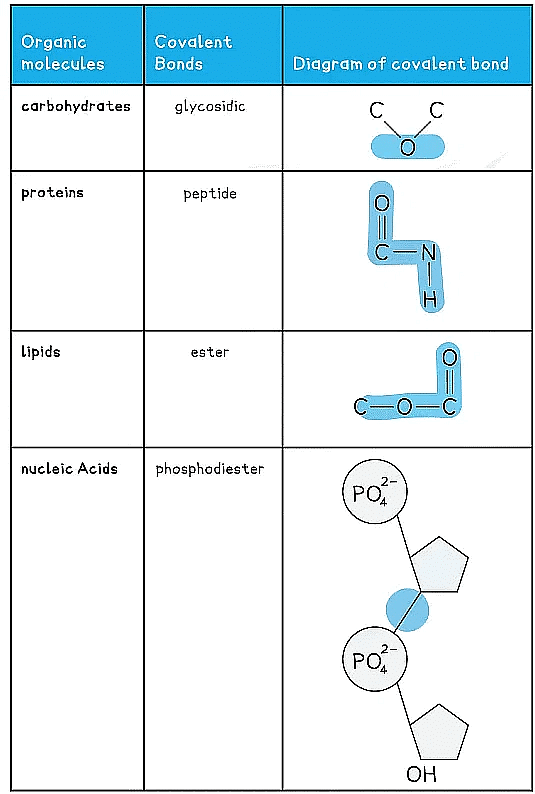
- A covalent bond is the sharing of two or more electrons between two atoms
- The electrons can be shared equally forming a nonpolar covalent bond or unequally (where an atom can be more electronegative δ-) to form a polar covalent bond
- Generally each atom will form a certain number of covalent bonds due to the number of free electrons in the outer orbital e.g. H = 1 bond, C = 4 bonds
- Covalent bonds are very stable as high energies are required to break the bonds
- Multiple pairs of electrons can be shared forming double bonds (e.g. unsaturated fats C=C) or triple bonds

Different types of covalent bonds
- When two monomers are close enough that their outer orbitals overlap this results in their electrons being shared and a covalent bond forming. If more monomers are added then polymerisation occurs (and / or a macromolecule forms)
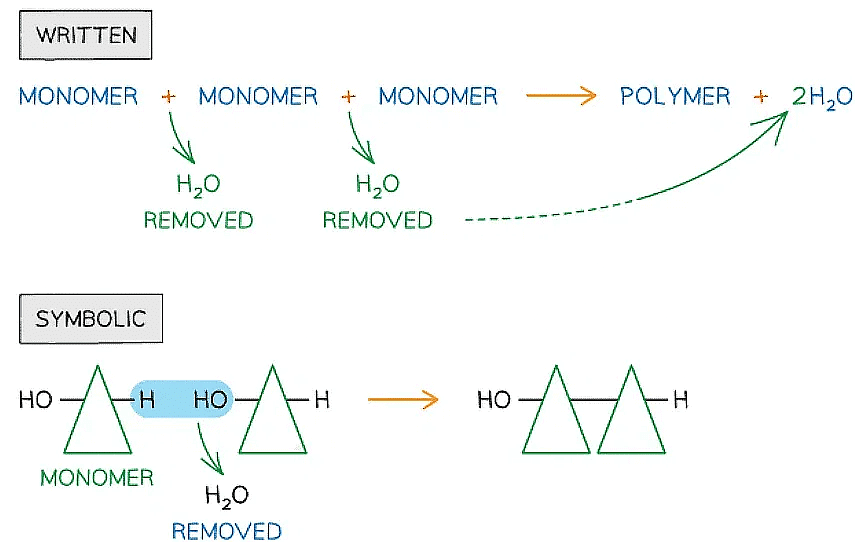
Condensation
- Also known as dehydration synthesis (‘to put together while losing water’)
- A condensation reaction occurs when monomers combine together by covalent bonds to form polymers (polymerisation) or macromolecules (lipids) and water is removed

Written and symbolic illustrations of the removal of water to form a covalent bond between two or more monomers during a condensation reaction
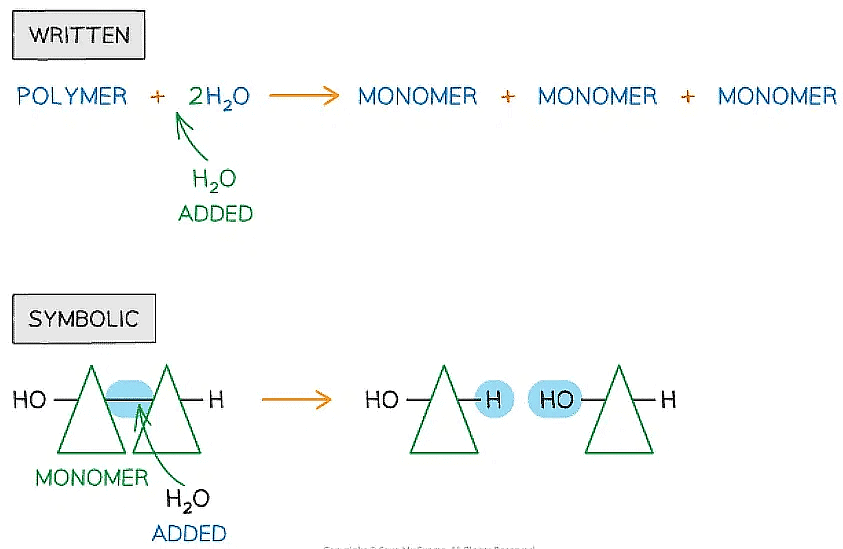
Hydrolysis
- Hydrolysis means ‘lyse’ (to break) and ‘hydro’ (with water)
- In the hydrolysis of polymers, covalent bonds are broken when water is added

Written and symbolic illustrations of the addition of water to break down covalent bond/s during a hydrolysis reaction
Covalent Bonds in Organic Molecules Table
Monosaccharides: Common Examples
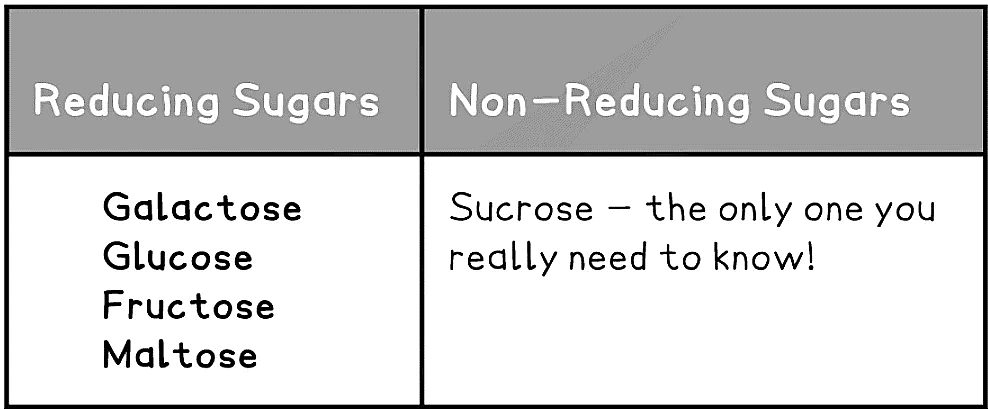
- Sugars can be classified as reducing or non-reducing; this classification is dependent on their ability to donate electrons
- Reducing sugars can donate electrons (the carbonyl group becomes oxidised), the sugars become the reducing agent
- Thus reducing sugars can be detected using Benedict’s test as they reduce the soluble copper sulphate to insoluble brick-red copper oxide
- Examples of reducing sugars include: glucose, fructose and galactose
- Fructose and galactose have the same molecular formula as glucose however they have a different structural formula
- The different arrangement of atoms in these monosaccharides gives them slightly different properties
- Non-reducing sugars cannot donate electrons, therefore they cannot be oxidised
- To be detected non-reducing sugars must first be hydrolysed to break the disaccharide into its two monosaccharides before a Benedict’s test can be carried out
- Example: sucrose

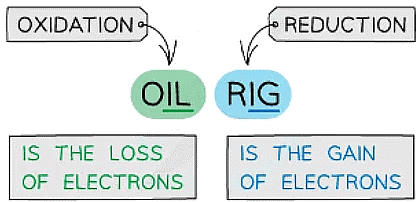
The mnemonic to remember the definitions for oxidation and reduction
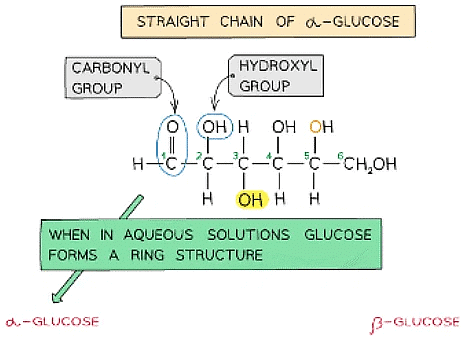
The Two Forms of Glucose
- The most well-known carbohydrate monomer is glucose ATP
- Glucose has the molecular formula C6H12O6
- Glucose is the most common monosaccharide and is of central importance to most forms of life
- There are different types of monosaccharide formed from molecules with varying numbers of carbon atom, for example:
- Trioses (3C) eg. glyceraldehyde
- Pentoses (5C) eg. ribose
- Hexoses (6C) eg. glucose
- Glucose exists in two structurally different forms – alpha (α) glucose and beta (β) glucose and is therefore known as an isomer
- This structural variety results in different functions between carbohydrates


- This structural variety results in different functions between carbohydrates
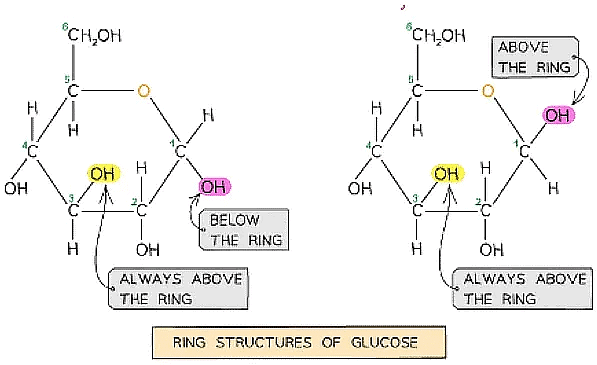
Straight chain and ring structural formula of alpha & beta glucose
- Different polysaccharides are formed from the two isomers of glucose
Structure of Polysaccharides Table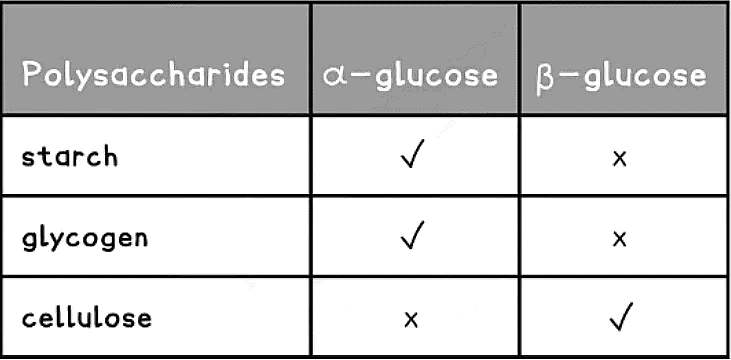
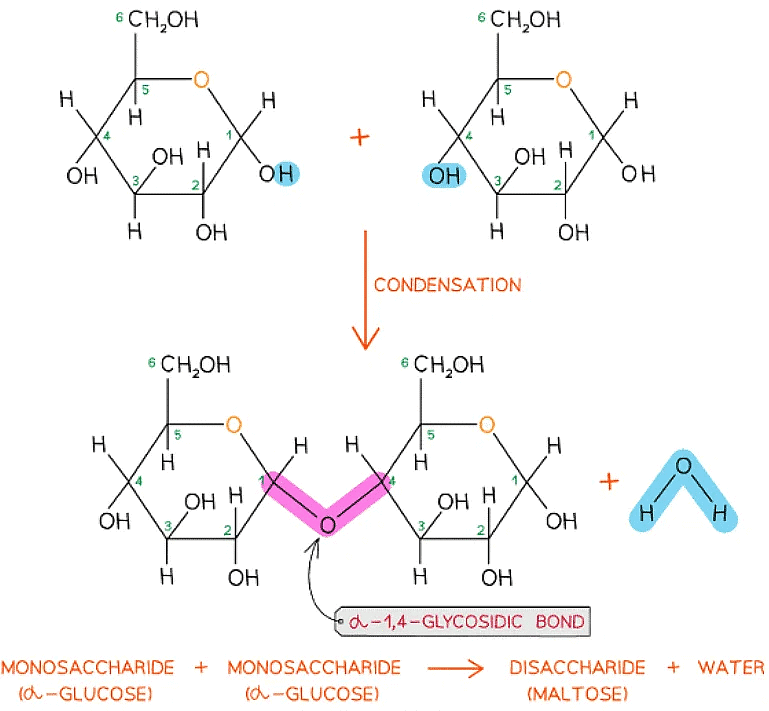
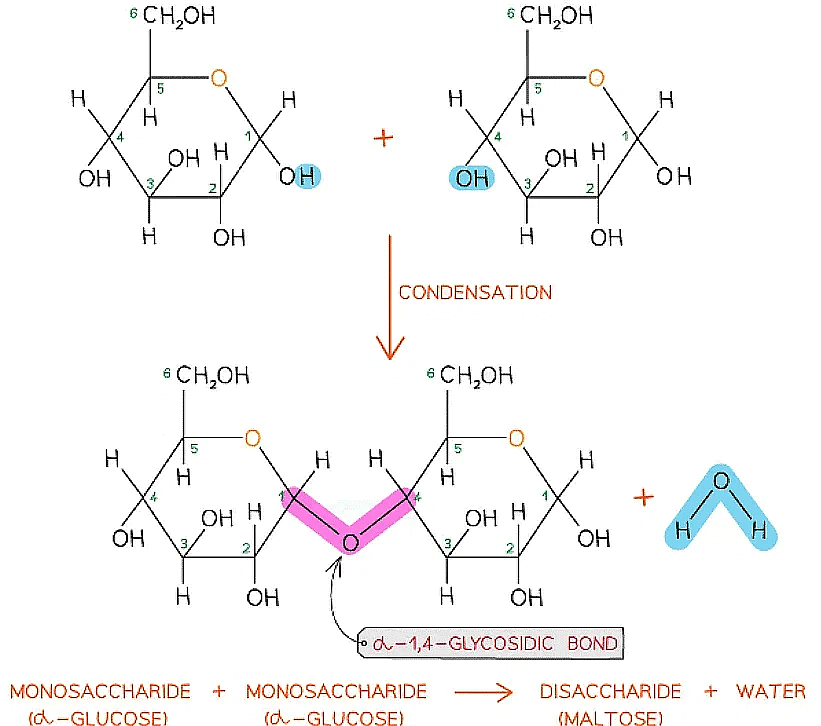
Forming the Glycosidic Bond
- To make monosaccharides more suitable for transport, storage and to have less influence on a cell’s osmolarity, they are bonded together to form disaccharides and polysaccharides
- Disaccharides and polysaccharides are formed when two hydroxyl (-OH) groups (on different saccharides) interact to form a strong covalent bond called the glycosidic bond (the oxygen link that holds the two molecules together)
- Every glycosidic bond results in one water molecule being removed, thus glycosidic bonds are formed by condensation

The formation of a glycosidic bond by condensation between two monosaccharides (glucose) to form a disaccharide (maltose)
- Each glycosidic bond is catalysed by enzymes specific to which OH groups are interacting
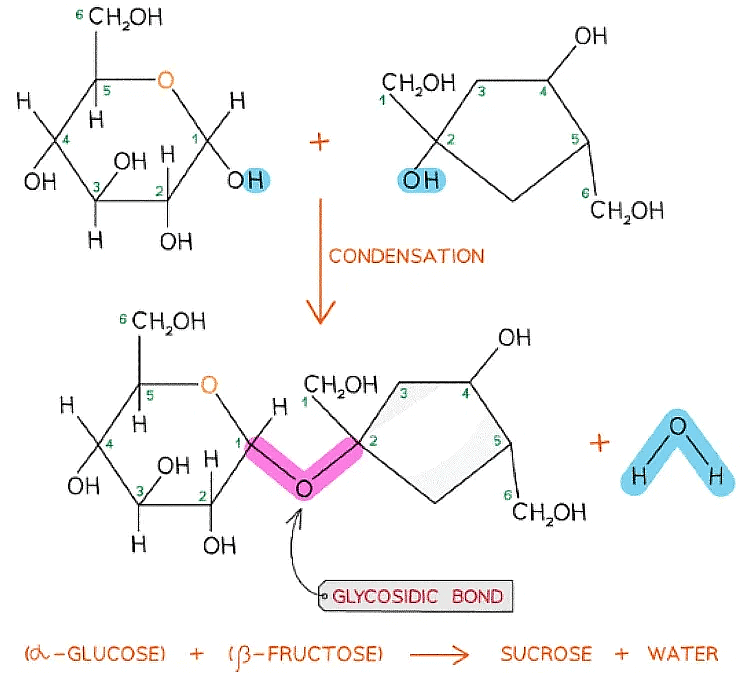
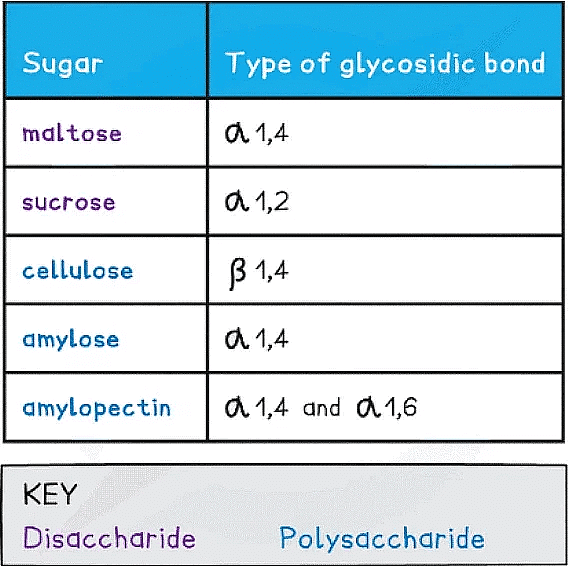
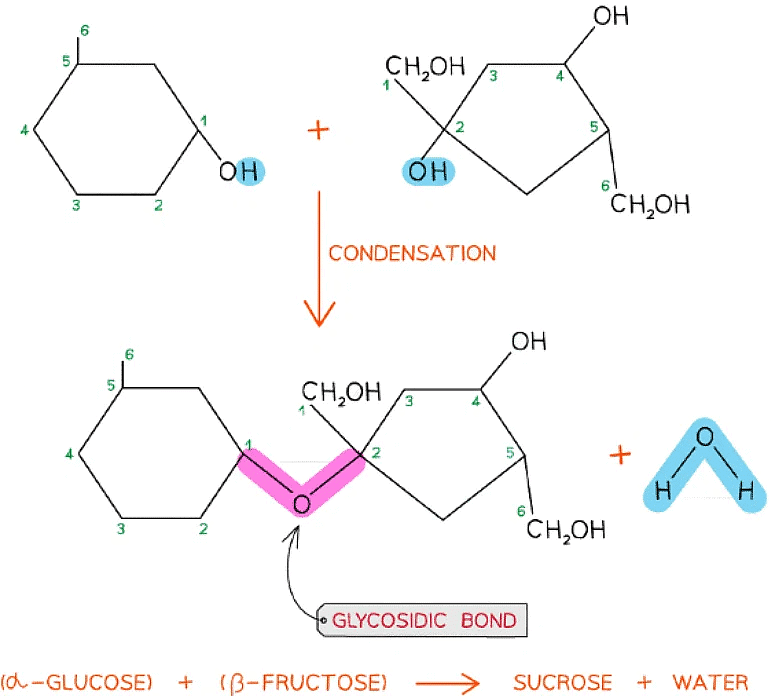
- As there are many different monosaccharides this results in different types of glycosidic bonds forming (e.g maltose has a α-1,4 glycosidic bond and sucrose has a α-1,2 glycosidic bond)

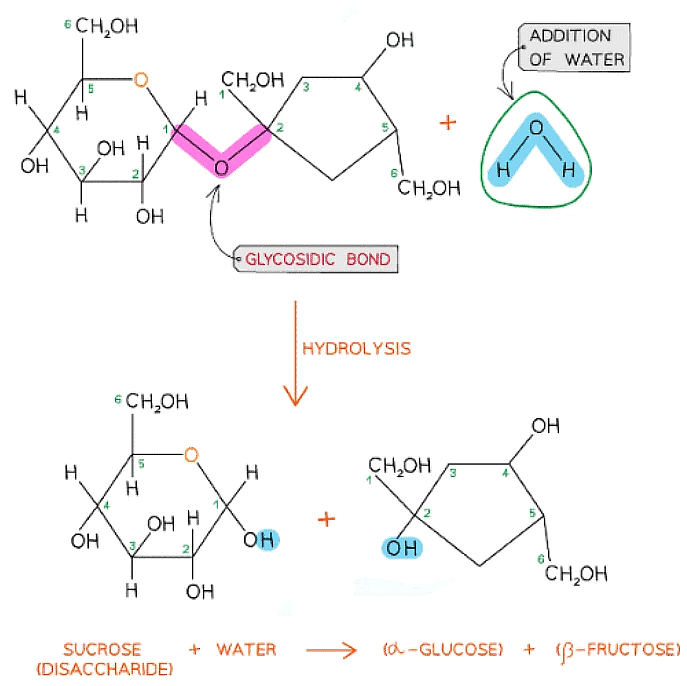
The formation of a glycosidic bond by condensation between α-glucose and β-fructose to form a disaccharide (sucrose)
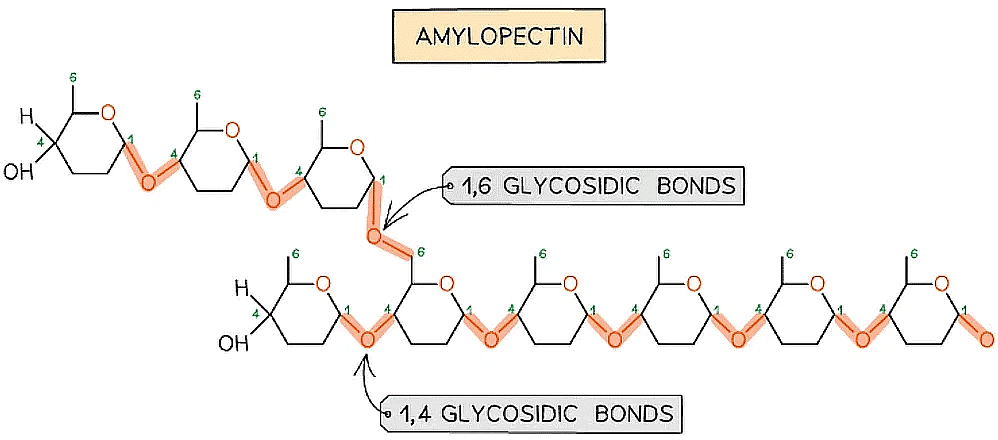 The formation of glycosidic bonds to create a polysaccharide (amylopectin)
The formation of glycosidic bonds to create a polysaccharide (amylopectin)
Types of Glycosidic Bonds Table
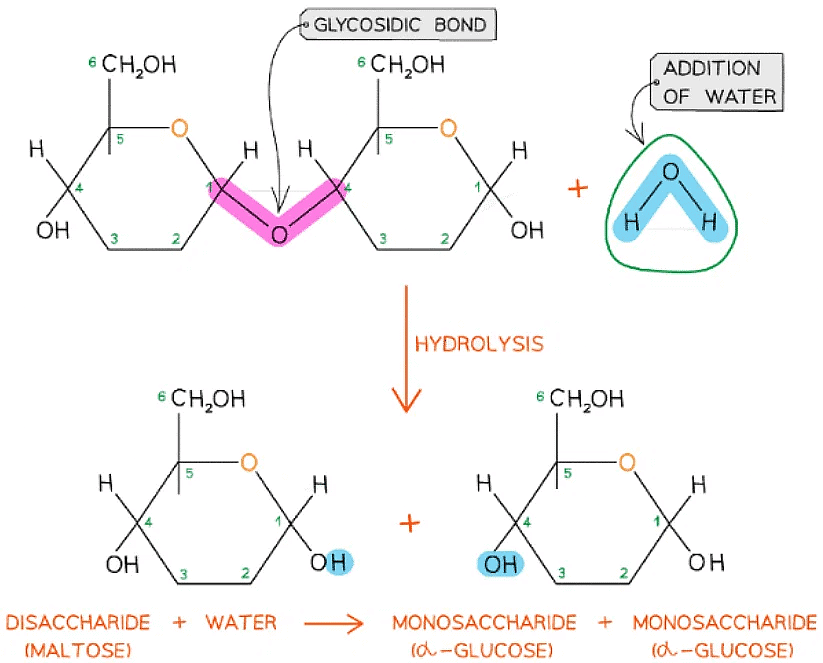
Breaking the Glycosidic Bond
- The glycosidic bond is broken when water is added in a hydrolysis (meaning ‘hydro’ - with water and ‘lyse’ - to break) reaction
- Disaccharides and polysaccharides are broken down in hydrolysis reactions
- Hydrolytic reactions are catalysed by enzymes, these are different to those present in condensation reactions
- Examples of hydrolytic reactions include the digestion of food in the alimentary tract and the breakdown of stored carbohydrates in muscle and liver cells for use in cellular respiration

Glycosidic bonds are broken by the addition of water in a hydrolysis reaction
- Sucrose is a non-reducing sugar which gives a negative result in a Benedict’s test. When sucrose is heated with hydrochloric acid this provides the water that hydrolyses the glycosidic bond resulting in two monosaccharides that will produce a positive Benedict's test

A molecule of glucose and a molecule of fructose are formed when one molecule of sucrose is hydrolysed; the addition of water to the glycosidic bond breaks it
Chromatography: Monosaccharides
- Chromatography is a technique that can be used to separate a mixture into its individual components
- Chromatography relies on differences in the solubility of the different chemicals (called ‘solutes’) within a mixture
- All chromatography techniques use two phases:
- The mobile phase
- The stationary phase
- The components in the mixture separate as the mobile phase travels over the stationary phase
- Differences in the solubility of each component in the mobile phase which affects how far each component can travel
- Those components with higher solubility will travel further than the others
- This is because they spend more time in the mobile phase and are thus carried further up the paper than the less soluble components
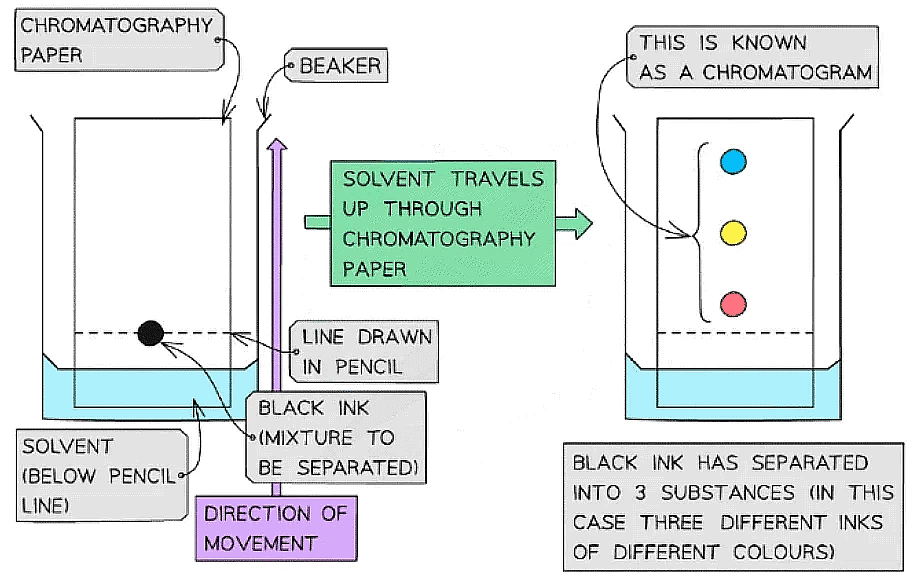
Paper Chromatography
- Paper chromatography is one specific form of chromatography
- In paper chromatography:
- The mobile phase is the solvent in which the sample molecules can move, which in paper chromatography is a liquid e.g. water or ethanol
- The stationary phase in paper chromatography is the chromatography paper
Paper chromatography method
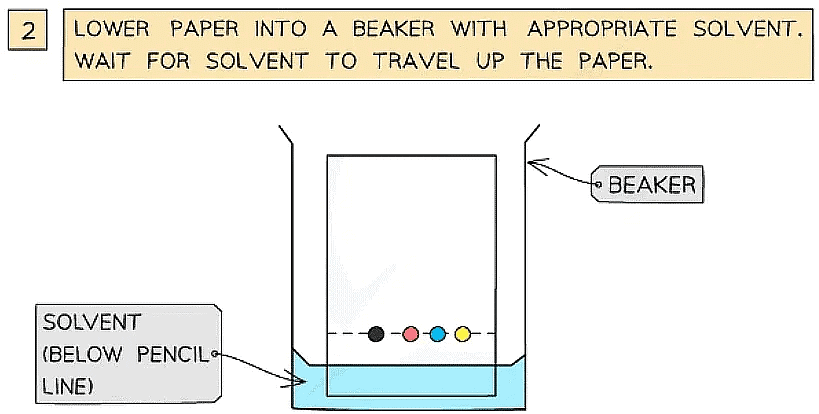
- A spot of the mixture (that you want to separate) is placed on chromatography paper and left to dry
- The chromatography paper is then suspended in a solvent
- As the solvent travels up through the chromatography paper, the different components within the mixture begin to move up the paper at different speeds
- Larger molecules move slower than smaller ones
- This causes the original mixture to separate out into different spots or bands on the chromatography paper
- This produces what is known as a chromatogram

An example of a chromatogram that has been produced by using paper chromatography to separate a spot of ink
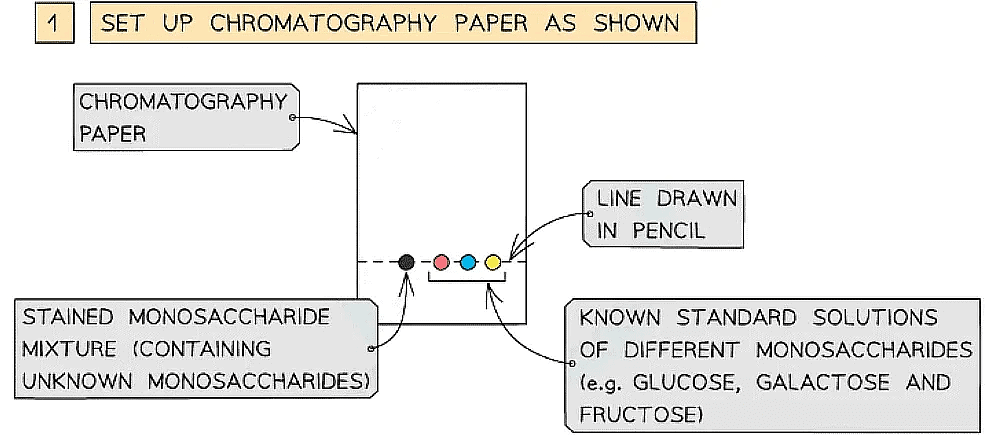
Using chromatography to separate a mixture of Monosaccharides
- Paper chromatography can be used to separate a mixture of monosaccharides
- Mixtures containing coloured molecules, such as ink or chlorophyll, do not have to be stained as they are already coloured
- Mixtures of colourless molecules, such as a mixture of monosaccharides, have to be stained first
- A spot of the stained monosaccharide sample mixture is placed on a line at the bottom of the chromatography paper
- Spots of known standard solutions of different monosaccharides are then placed on the line beside the sample spot
- The chromatography paper is then suspended in a solvent
- As the solvent travels up through the chromatography paper, the different monosaccharides within the mixture separate out at different distances from the line
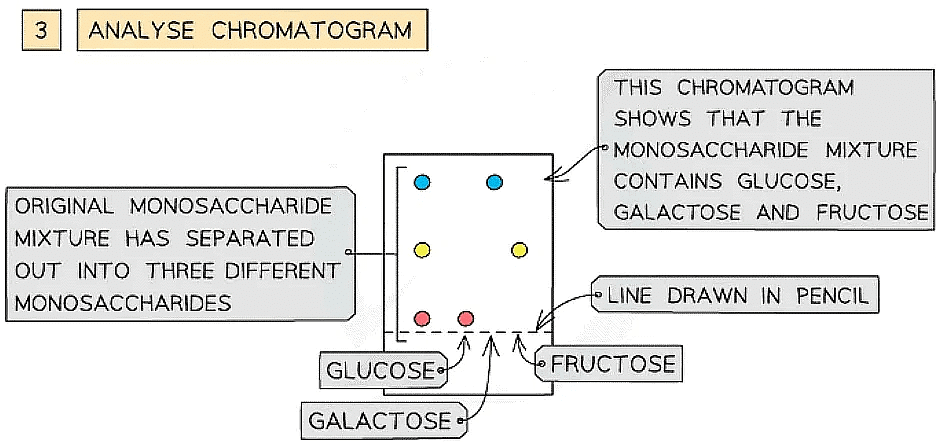
- The unknown monosaccharides can then be identified by comparing and matching them with the chromatograms of the known standard solutions of different monosaccharides
- If a spot from the monosaccharide sample mixture is at the same distance from the line as a spot from one of the known standard solutions, then the mixture must contain this monosaccharide



- If a spot from the monosaccharide sample mixture is at the same distance from the line as a spot from one of the known standard solutions, then the mixture must contain this monosaccharide
How chromatography can be used to separate a mixture of monosaccharides and identify the individual components
Disaccharides: Common Examples
- Monosaccharides can join together via condensation reactions to form disaccharides
- A condensation reaction is one in which two molecules join together via the formation of a new chemical bond, with a molecule of water being released in the process
- The new chemical bond that forms between two monosaccharides is known as a glycosidic bond
- To calculate the chemical formula of a disaccharide, you add all the carbons, hydrogens and oxygens in both monomers then subtract 2x H and 1x O (for the water molecule lost)
- Common examples of disaccharides include:
- Maltose (the sugar formed in the production and breakdown of starch)
- Sucrose (the main sugar produced in plants)
- Lactose (a sugar found only in milk)
- All three of the common examples above have the formula C12H22O11
Common Disaccharides and their Monosaccharide Monomers Table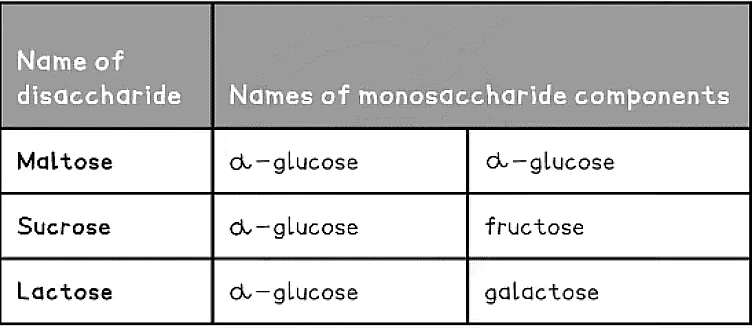
The disaccharide maltose is formed from two α-glucose monomers (sub-units)
Starch & Glycogen: Structures & Functions
- Starch and glycogen are polysaccharides
- Polysaccharides are macromolecules that are polymers formed by many monosaccharides joined by glycosidic bonds in a condensation reaction to form chains. These chains may be:
- Branched or unbranched
- Folded (making the molecule compact which is ideal for storage eg. starch and glycogen)
- Straight (making the molecules suitable to construct cellular structures e.g. cellulose) or coiled
- Starch and glycogen are storage polysaccharides because they are:
- Compact (so large quantities can be stored)
- Insoluble (so will have no osmotic effect, unlike glucose which would lower the water potential of a cell causing water to move into cells, cells would then have to have thicker cell walls - plants or burst if they were animal cells)
Starch
- Starch is the storage polysaccharide of plants. It is stored as granules in plastids (e.g. chloroplasts)
- Due to the many monomers in a starch molecule, it takes longer to digest than glucose
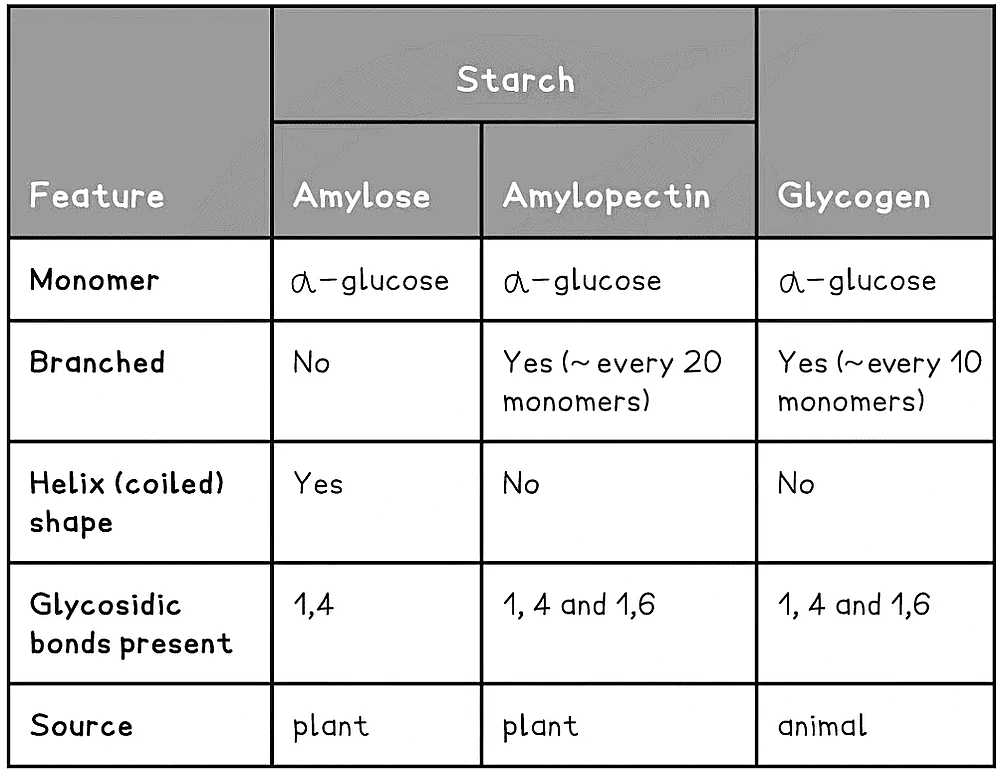
- Starch is constructed from two different polysaccharides:
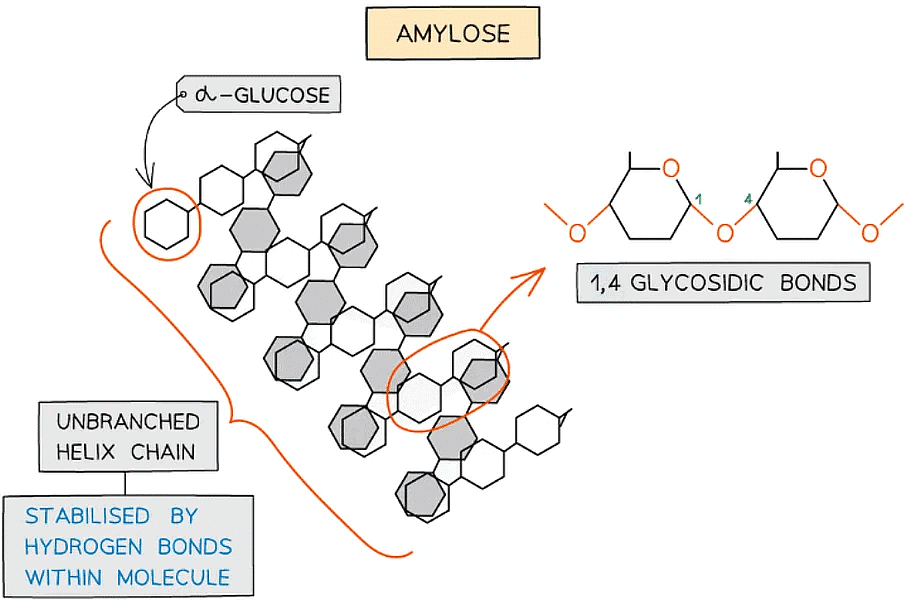
- Amylose (10 - 30% of starch)
- Unbranched helix-shaped chain with 1,4 glycosidic bonds between α-glucose molecules
- The helix shape enables it to be more compact and thus it is more resistant to digestion

Amylose – one of the two polysaccharides that is used to form starch (the storage polysaccharide in plants)
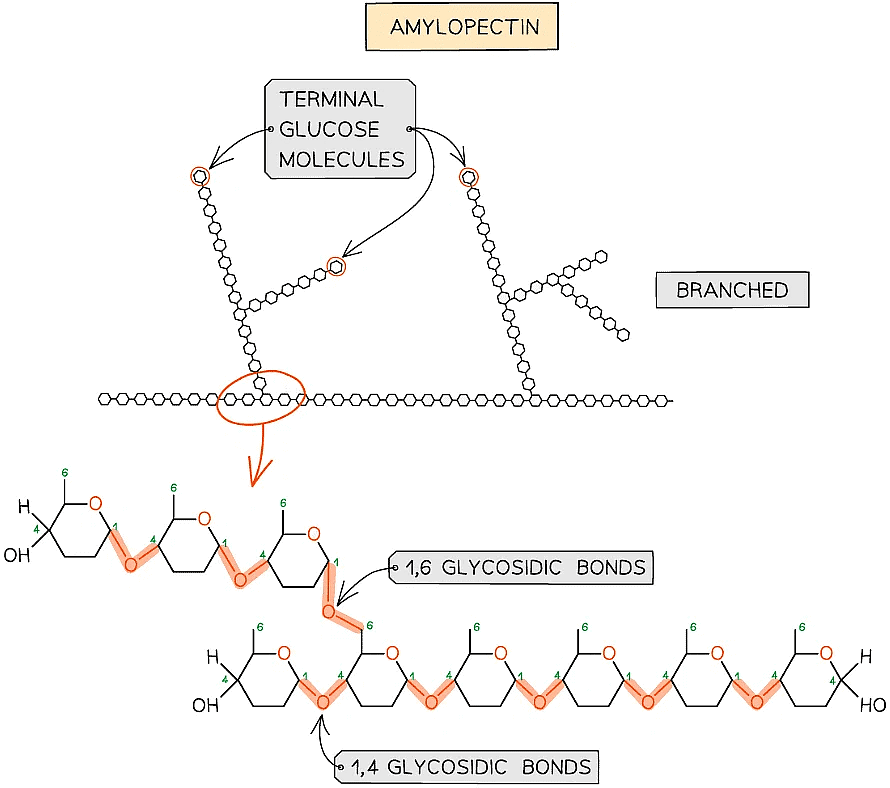
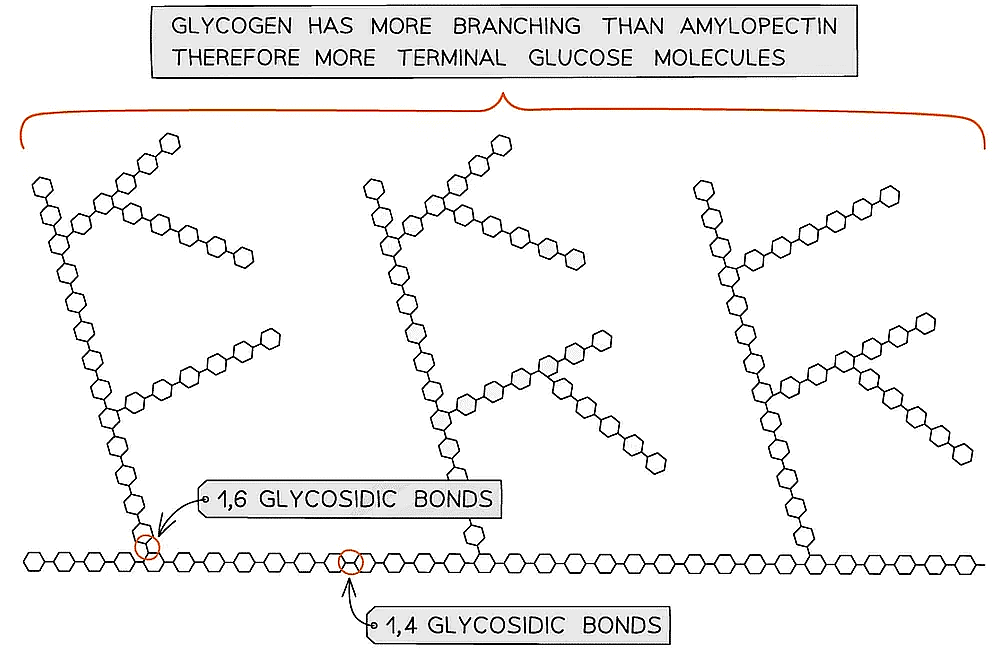
- Amylopectin (70 - 90% of starch)
- 1,4 glycosidic bonds between α-glucose molecules but also 1,6 glycosidic bonds form between glucose molecules creating a branched molecule
- The branches result in many terminal glucose molecules that can be easily hydrolysed for use during cellular respiration or added to for storage

Amylopectin – one of the two polysaccharides that is used to form starch (the storage polysaccharide in plants)
Glycogen
- Glycogen is the storage polysaccharide of animals and fungi, it is highly branched and not coiled
- Liver and muscles cells have a high concentration of glycogen, present as visible granules, as the cellular respiration rate is high in these cells (due to animals being mobile)
- Glycogen is more branched than amylopectin making it more compact which helps animals store more
- The branching enables more free ends where glucose molecules can either be added or removed allowing for condensation and hydrolysis reactions to occur more rapidly – thus the storage or release of glucose can suit the demands of the cell

Glycogen, the highly branched molecule used as a storage polysaccharide in animals and fungi
Summary of Storage Polysaccharides Table
Cellulose: Structure & Function
- Cellulose is a polysaccharide
- Polysaccharides are macromolecules that are polymers formed by many monosaccharides joined by glycosidic bonds in a condensation reaction to form chains. These chains may be:
- Branched or unbranched
- Folded (making the molecule compact which is ideal for storage, eg. starch and glycogen)
- Straight (making the molecules suitable to construct cellular structures, eg. cellulose) or coiled
- Polysaccharides are insoluble in water
Cellulose – structure
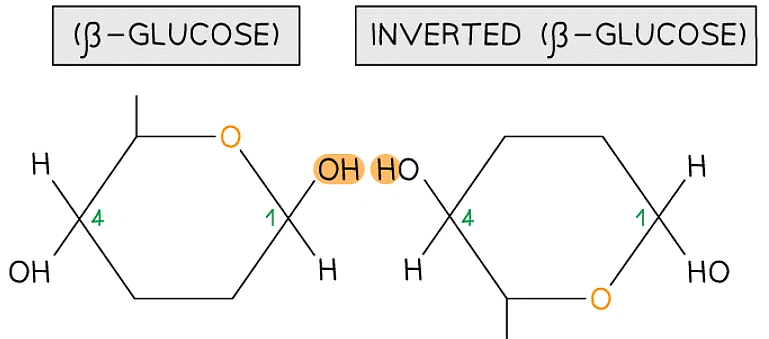
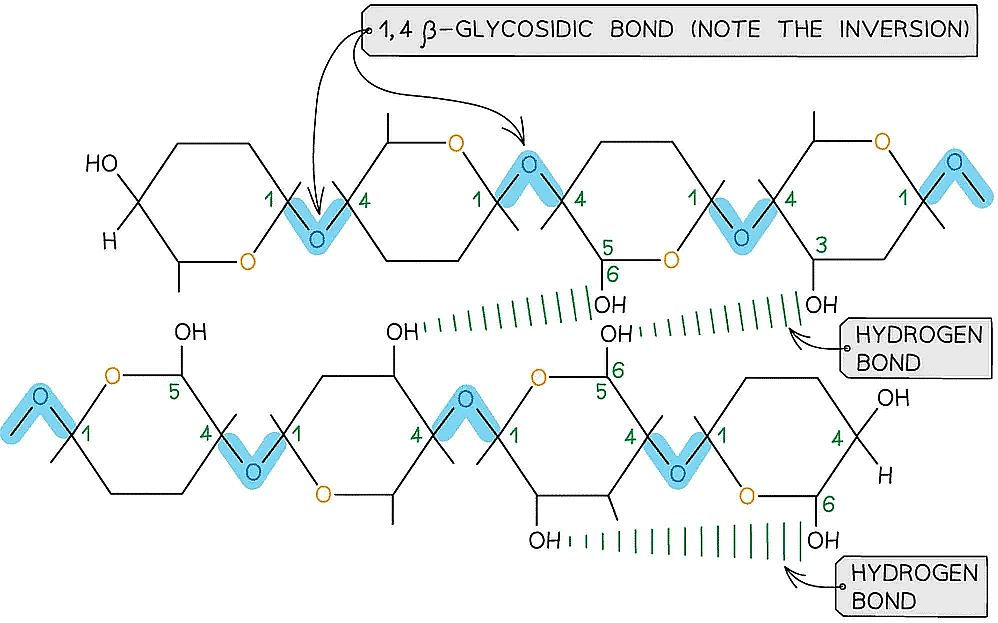
- Is a polymer consisting of long chains of β-glucose joined together by 1,4 glycosidic bonds
- As β-glucose is an isomer of α-glucose to form the 1,4 glycosidic bonds consecutive β-glucose molecules must be rotated 180° to each other

To form the 1,4 glycosidic bond between two β-glucose molecules, the glucose molecules must be rotated to 180° to each other
- Due to the inversion of the β-glucose molecules many hydrogen bonds form between the long chains giving cellulose it’s strength

Cellulose is used as a structural component due to the strength it has from the many hydrogen bonds that form between the long chains of β-glucose molecules
Cellulose – function
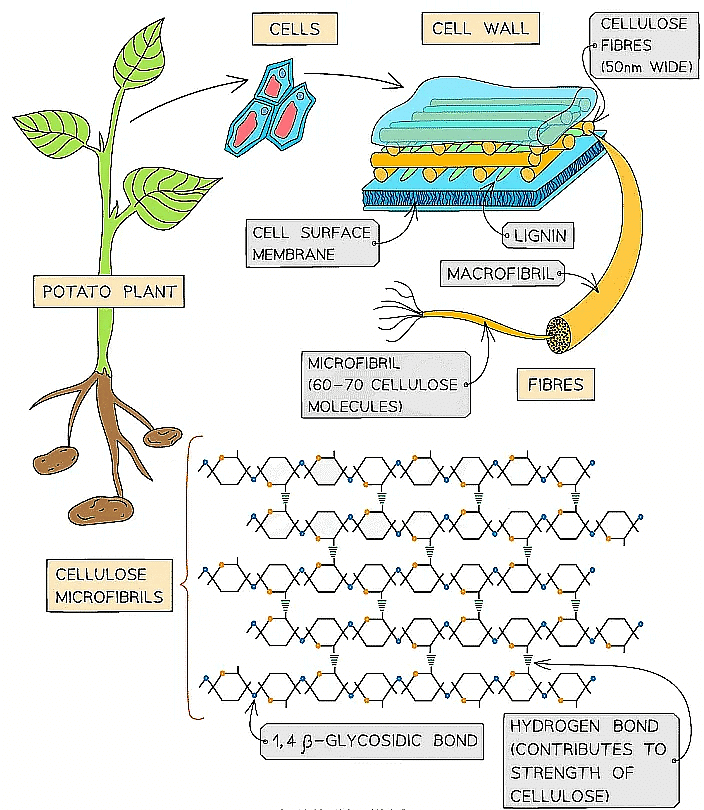
- Cellulose is the main structural component of cell walls due to its strength which is a result of the many hydrogen bonds found between the parallel chains of microfibrils
- The high tensile strength of cellulose allows it to be stretched without breaking which makes it possible for cell walls to withstand turgor pressure
- The cellulose fibres and other molecules (eg. lignin) found in the cell wall form a matrix which increases the strength of the cell walls
- The strengthened cell walls provides support to the plant
- Cellulose fibres are freely permeable which allows water and solutes to leave or reach the cell surface membrane
- As few organisms have the enzyme (cellulase) to hydrolyse cellulose it is a source of fibre

The strength and insolubility of cellulose fibres means it is a suitable molecule to construct cell walls
 |
Download the notes
Biological Molecules: Carbohydrates
|
Download as PDF |
Biochemical Tests: Sugars & Starch
- There are a number of tests that can be carried out quickly and easily in a lab to determine if a sample contains a certain type of sugar
- The following tests are qualitative - they do not give a quantitative value as to how much of each type of molecule may be present in a sample
- Sugars can be classified as reducing or non-reducing; this classification is dependent on their ability to donate electrons (a reducing sugar that is able to donate electrons is itself oxidised)
- OILRIG in Chemistry
The Benedict’s test for reducing sugars
- Benedict’s reagent is a blue solution that contains copper (II) sulfate ions (CuSO4 ); in the presence of a reducing sugar copper (I) oxide forms
- Copper (I) oxide is not soluble in water, so it forms a precipitate
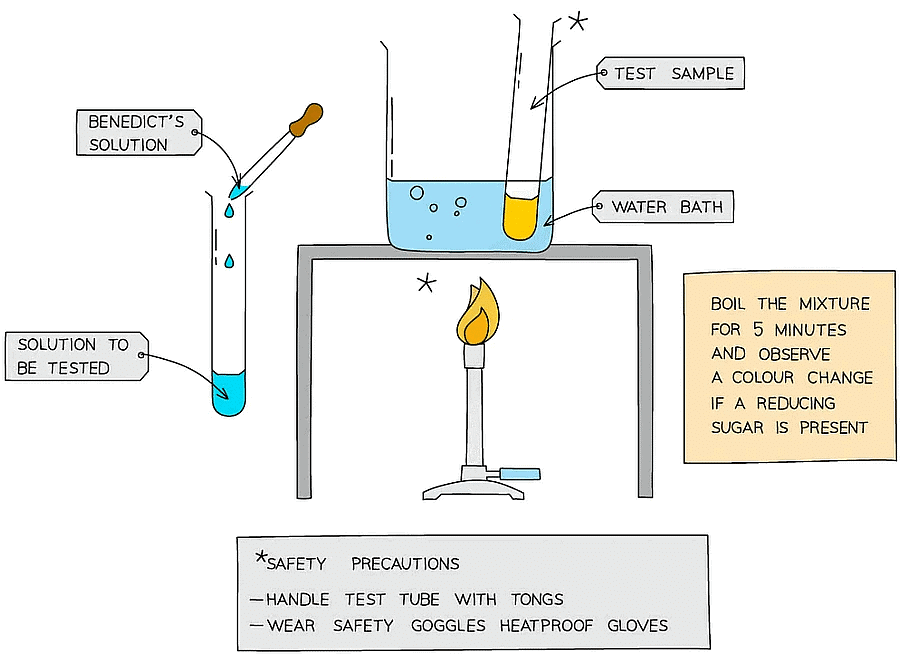
Method
- Add Benedict's reagent (which is blue as it contains copper (II) sulfate ions) to a sample solution in a test tube
- Heat the test tube in a water bath or beaker of water that has been brought to a boil for a few minutes
- If a reducing sugar is present, a coloured precipitate will form as copper (II) sulfate is reduced to copper (I) oxide which is insoluble in water
- It is important that an excess of Benedict’s solution is used so that there is more than enough copper (II) sulfate present to react with any sugar present
- A positive test result is a colour change somewhere along a colour scale from blue (no reducing sugar), through green, yellow and orange (low to medium concentration of reducing sugar) to brown/brick-red (a high concentration of reducing sugar)
- This test is semi-quantitative as the degree of the colour change can give an indication of how much (the concentration of) reducing sugar present

- This test is semi-quantitative as the degree of the colour change can give an indication of how much (the concentration of) reducing sugar present
Reducing & Non-reducing Sugars Table
To test for non-reducing sugars:
- Add dilute hydrochloric acid to the sample and heat in a water bath that has been brought to the boil
- Neutralise the solution with sodium hydrogencarbonate
- Use a suitable indicator (such as red litmus paper) to identify when the solution has been neutralised, and then add a little more sodium hydrogencarbonate as the conditions need to be slightly alkaline for the Benedict’s test to work
- Then carry out the Benedict’s test as normal; add Benedict’s reagent to the sample and heat in a water bath that has been boiled – if a colour change occurs, a reducing sugar is present
Explanation
- The addition of acid will hydrolyse any glycosidic bonds present in any carbohydrate molecules
- The resulting monosaccharides left will have an aldehyde or ketone functional group that can donate electrons to copper (II) sulfate (reducing the copper), allowing a precipitate to form
The iodine test for starch
- To test for the presence of starch in a sample, add a few drops of orange/brown iodine in potassium iodide solution to the sample
- The iodine is in potassium iodide solution as iodine is insoluble in water
- If starch is present, iodide ions in the solution interact with the centre of starch molecules, producing a complex with a distinctive blue-black colour
- This test is useful in experiments for showing that starch in a sample has been digested by enzymes
Finding the Concentration of Glucose
- Benedict’s solution can be used to carry out a semi-quantitative test on a reducing sugar solution to determine the concentration of reducing sugar present in the sample
- It is important that an excess of Benedict’s solution is used so that there is more than enough copper (II) sulfate present to react with any sugar present
- The intensity of any colour change seen relates to the concentration of reducing sugar present in the sample
- A positive test is indicated along a spectrum of colour from green (low concentration) to brick-red (high concentration of reducing sugar present)
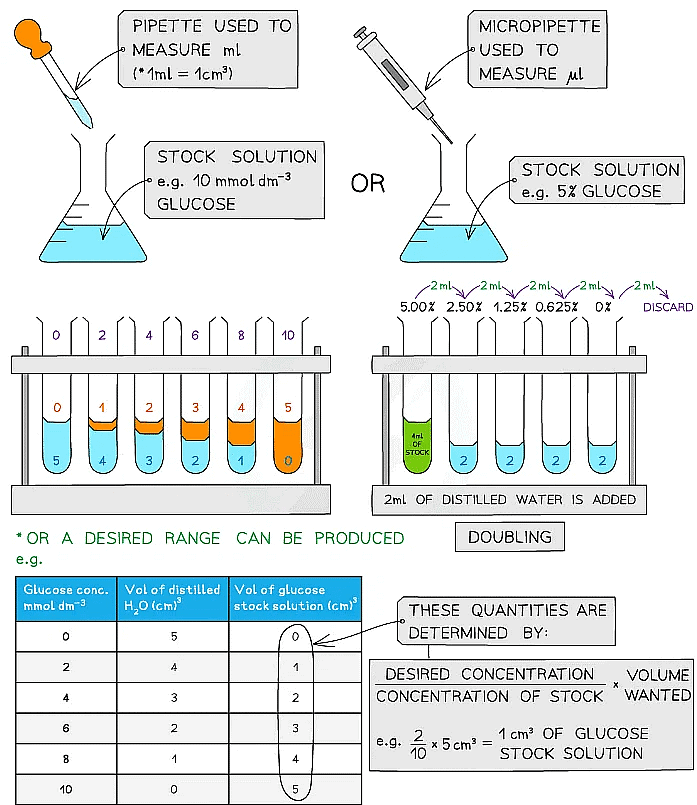
- A semi-quantitative test can be carried out by setting up standard solutions with known concentrations of a reducing sugar (such as glucose)
- These solutions should be set up using a serial dilution of an existing stock solution
- Each solution is then treated in the same way: add the same volume of Benedict’s solution to each sample and heat in a water bath that has been boiled (ideally at the same temperature each time) for a set time (5 minutes or so) to allow colour changes to occur
- It is important to ensure that an excess of Benedict’s solution is used
- Any colour change observed for each solution of a known concentration in that time can be attributed to the concentration of reducing sugar present in that solution
- The same procedure is carried out on a sample with an unknown concentration of reducing sugar which is then compared to the stock solution colours to estimate the concentration of reducing sugar present
Alterations
- It is also possible to standardise this test but instead of waiting a fixed amount of time for a range of colours to be observed, time how long it takes for the first colour change to occur (blue to green)
- The higher the concentration of reducing sugar in a sample, the less time it would take for a colour change to be observed
- To avoid issues with human interpretation of colour, a colourimeter could be used to measure the absorbance or transmission of light through the sugar solutions of known concentration to establish a range of values that an unknown sample can be compared against a calibration curve
Serial dilutions
- Serial dilutions are created by taking a series of dilutions of a stock solution. The concentration decreases by the same quantity between each test tube
- They can either be ‘doubling dilutions’ (where the concentration is halved between each test tube) or a desired range (e.g. 0, 2, 4, 6, 8, 10 mmol dm-3)
- Serial dilutions are completed to create a standard to compare unknown concentrations against
- The comparison can be:
- Visual
- Measured through a calibration/standard curve
- Measured using a colourimeter
- They can be used when:
- Counting bacteria or yeast populations
- Determining unknown glucose, starch, protein concentrations

Making serial dilutions
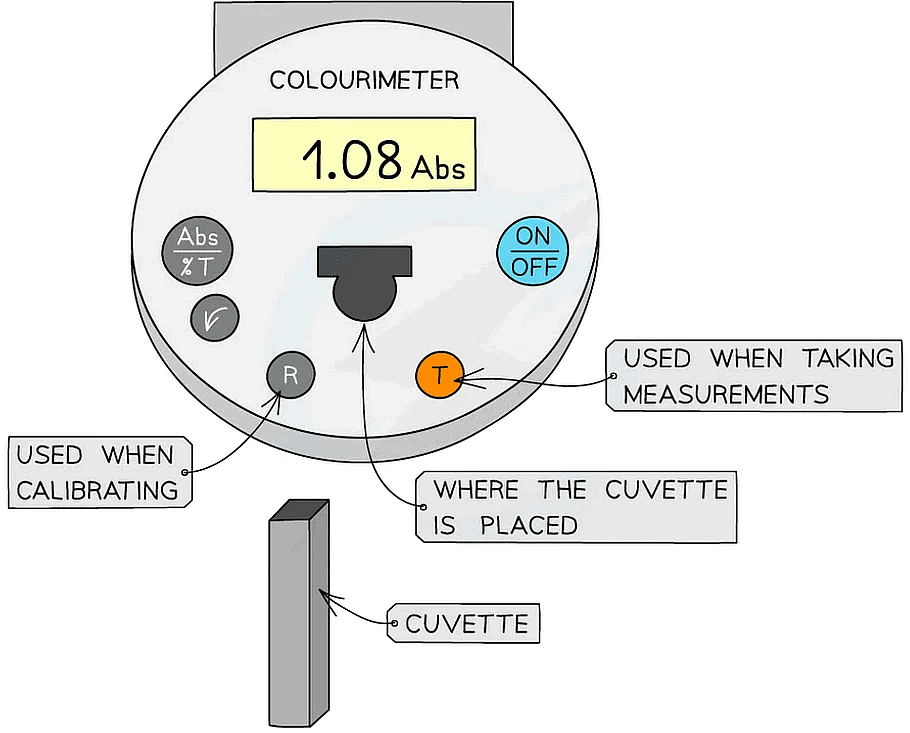
Colourimeter
- A colourimeter is an instrument that beams a specific wavelength (colour) of light through a sample and measures how much of this light is absorbed (arbitrary units)
- They provide a quantitative measurement
- They contain different wavelengths or colour filters (depends on the model of colourimeter), so that a suitable colour can be shone through the sample and will not get absorbed. This colour will be the contrasting colour (eg. a red sample should have green light shone through)
- Remember that a sample will look red as that wavelength of light is being reflected but the other wavelengths will be absorbed
- Colourimeters must be calibrated before taking measurements
- This is completed by placing a blank into the colourimeter and taking a reference, it should read 0 (that is, no light is being absorbed)
- This step should be repeated periodically whilst taking measurements to ensure that the absorbance is still 0
- The results can then be used to plot a calibration or standard curve
- Absorbance against the known concentrations can be used
- Unknown concentrations can then be determined from this graph

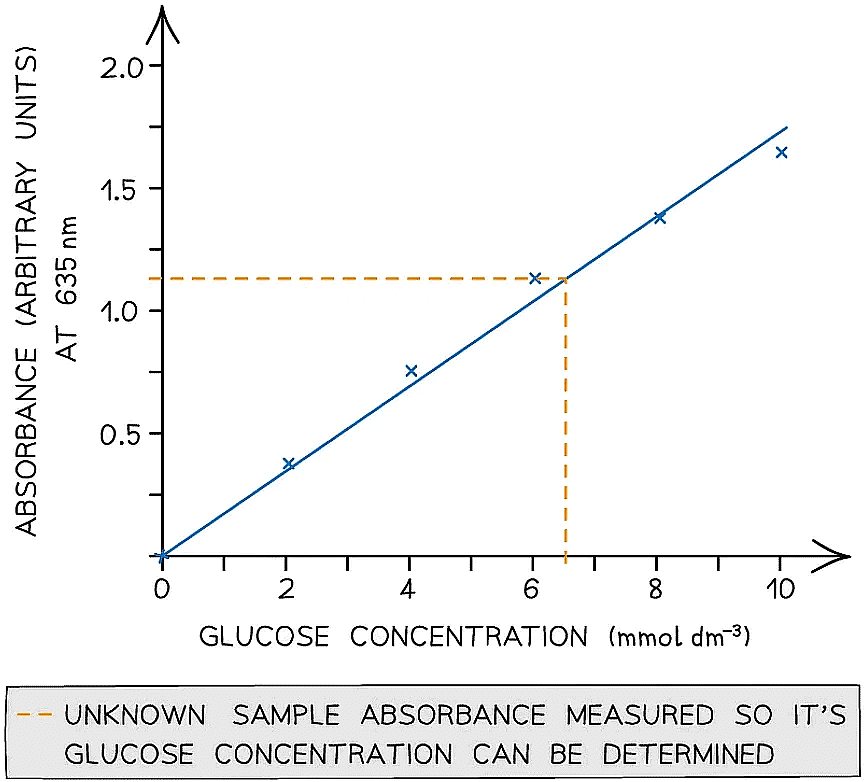 A colourimeter is used to obtain quantitative data that can be plotted to create a calibration curve to be used to find unknown concentrations
A colourimeter is used to obtain quantitative data that can be plotted to create a calibration curve to be used to find unknown concentrations
|
122 videos|161 docs|138 tests
|