Grade 12 Exam > Grade 12 Notes > Biology for Grade 12 > The Structure of ATP
The Structure of ATP | Biology for Grade 12 PDF Download
Introduction
- All organisms require a constant supply of energy to maintain their cells and stay alive
- This energy is required:
- In anabolic reactions – building larger molecules from smaller molecules
- To move substances across the cell membrane (active transport) or to move substances within the cell
- In animals, energy is required:
- For muscle contraction – to coordinate movement at the whole-organism level
- In the conduction of nerve impulses, as well as many other cellular processes
- In all known forms of life, ATP from respiration is used to transfer energy in all energy-requiring processes in cells
- This is why ATP is known as the universal energy currency
- Adenosine Triphosphate (ATP) is a nucleotide
- The monomers of DNA and RNA are also nucleotide
ATP
- Adenosine triphosphate (ATP) is the energy-carrying molecule that provides the energy to drive many processes inside living cells
- ATP is another type of nucleic acid and hence it is structurally very similar to the nucleotides that make up DNA and RNA
- It is a phosphorylated nucleotide
- Adenosine (a nucleoside) can be combined with one, two or three phosphate groups
- One phosphate group = adenosine monophosphate (AMP)
- Two phosphate groups = adenosine diphosphate (ADP)
- Three phosphate groups = adenosine triphosphate (ATP)
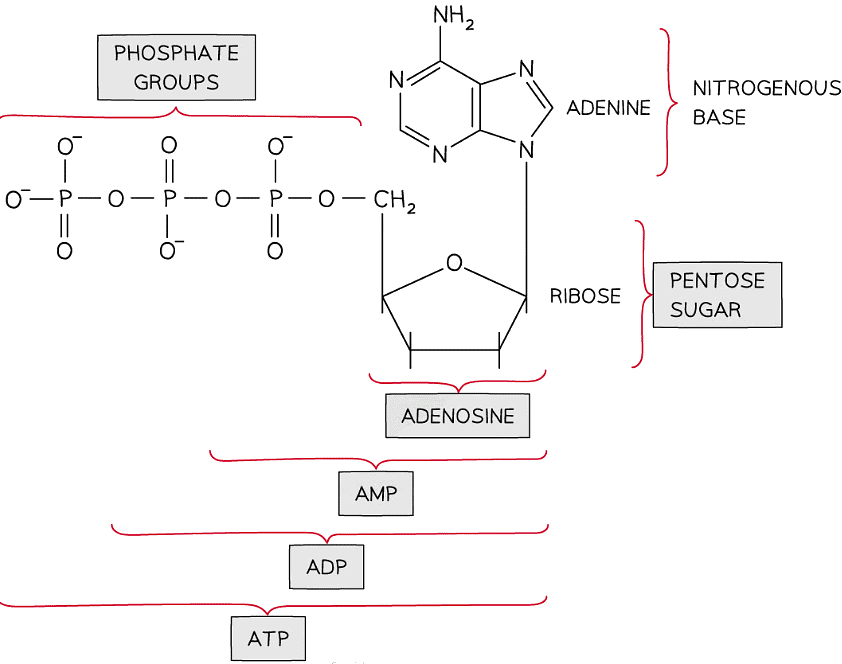
The structure of AMP, ADP and ATP
Hydrolysis & Synthesis of ATP
Hydrolysis of ATP
- Energy released during the reactions of respiration is transferred to the molecule adenosine triphosphate (ATP)
- ATP is a small and soluble molecule that provides a short-term store of chemical energy that cells can use to do work
- It is vital in linking energy-requiring and energy-yielding reactions
- ATP is described as a universal energy currency
- Universal: It is used in all organisms
- Currency: Like money, it can be used for different purposes (reactions) and is reused countless times
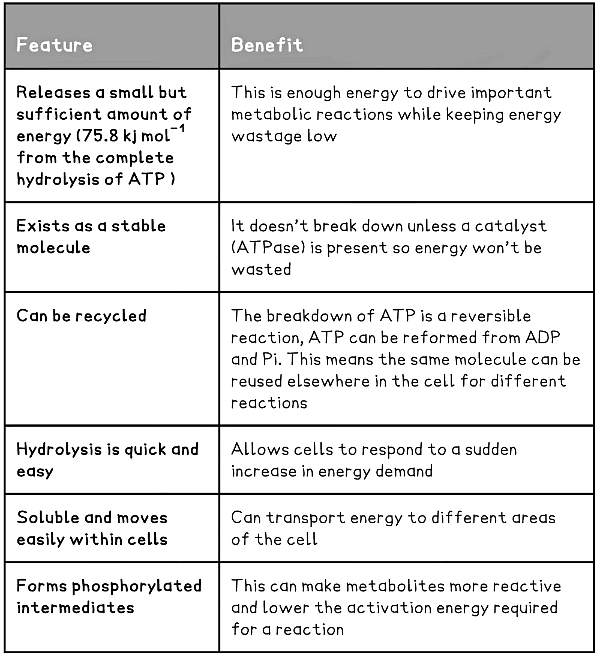
- The use of ATP as an ‘energy-currency’ is beneficial for many reasons:
- The hydrolysis of ATP can be carried out quickly and easily wherever energy is required within the cell by the action of just one enzyme, ATPase
- A useful (not too small, not too large) quantity of energy is released from the hydrolysis of one ATP molecule - this is beneficial as it reduces waste but also gives the cell control over what processes occur
- ATP is relatively stable at cellular pH levels
Hydrolysis of ATP
- Hydrolysis of ATP to adenosine diphosphate (ADP) and an inorganic phosphate group (Pi) is catalysed by the enzyme ATP hydrolase sometimes called 'ATPase'
- The hydrolysis of ATP can be coupled to energy-requiring reactions within cells such as:
- The active transport of ions up a concentration gradient
- Enzyme controlled reactions that require energy
- Muscle contraction and muscle fibre movement
- As ADP forms free energy is released that can be used for processes within a cell eg. DNA synthesis
- Removal of one phosphate group from ATP releases 30.8 kJ mol -1 of energy, forming ADP
- Removal of a second phosphate group from ADP also releases 30.8 kJ mol-1 of energy, forming AMP
- Removal of the third and final phosphate group from AMP releases 14.2 kJ mol-1 of energy, forming adenosine
- The inorganic phosphate released during the hydrolysis of ATP can be used to phosphorylate other compounds, often making them more reactive
Features of ATP Table
ATP Synthesis
- On average humans use more than 50 kg of ATP in a day but only have a maximum of ~ 200g of ATP in their body at any given time
- Organisms cannot build up large stores of ATP and it rarely passes through the cell surface membrane
- This means the cells must make ATP as and when they need it
- ATP is formed when ADP is combined with an inorganic phosphate (Pi) group by the enzyme ATP synthase
- This is an energy-requiring reaction
- Water is released as a waste product (therefore ATP synthesis is a condensation reaction)
Types of ATP synthesis
- ATP is made during the reactions of respiration and photosynthesis
- All of an animal's ATP comes from respiration
- ATP can be made in two different ways:
- Substrate-linked phosphorylation (occurs in the glycolysis stage of respiration)
- Chemiosmosis (occurs in the electron transport chain stage of respiration)
The Properties of Water
Water in Cells
- Water is of great biological importance. It is the medium in which all metabolic reactions take place in cells. Between 70% to 95% of the mass of a cell is water
- As 71% of the Earth’s surface is covered in water it is a major habitat for organisms
- Water is composed of atoms of hydrogen and oxygen. One atom of oxygen combines with two atoms of hydrogen by sharing electrons (covalent bonding)
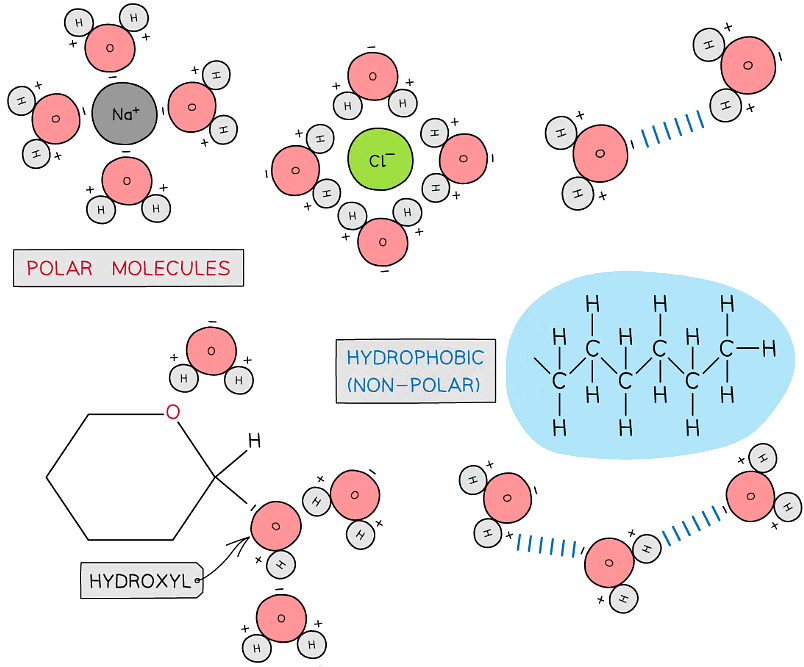
- Although water as a whole is electrically neutral the sharing of the electrons is uneven between the oxygen and hydrogen atoms
- The oxygen atom attracts the electrons more strongly than the hydrogen atoms, resulting in a weak negatively charged region on the oxygen atom (δ-) and a weak positively charged region on the hydrogen atoms(δ+), this also results in the asymmetrical shape
- This separation of charge due to the electrons in the covalent bonds being unevenly shared is called a dipole. When a molecule has one end that is negatively charged and one end that is positively charged it is also a polar molecule
- Water is a polar molecule
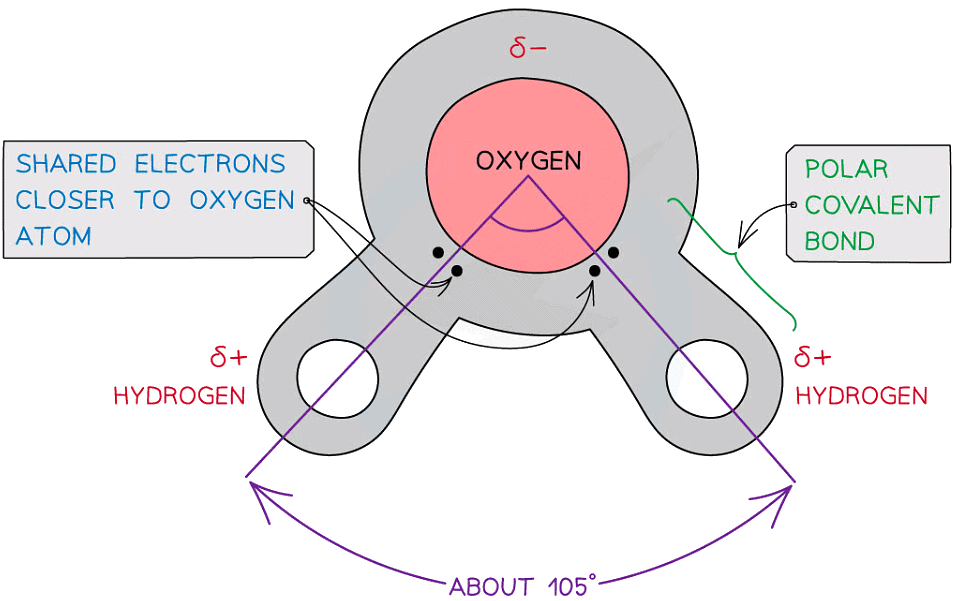
The covalent bonds of water make it a polar molecule
- Hydrogen bonds form between water molecules
- As a result of the polarity of water hydrogen bonds form between the positive and negatively charged regions of adjacent water molecules
- Hydrogen bonds are weak, when there are few, so they are constantly breaking and reforming. However when there are large numbers present they form a strong structure
- Hydrogen bonds contribute to the many properties water molecules have that make them so important to living organisms:
- An excellent solvent – many substances can dissolve in water
- A relatively high specific heat capacity
- A relatively high latent heat of vaporisation
- Water is less dense when a solid
- Water has high surface tension and cohesion
- It acts as a reagent
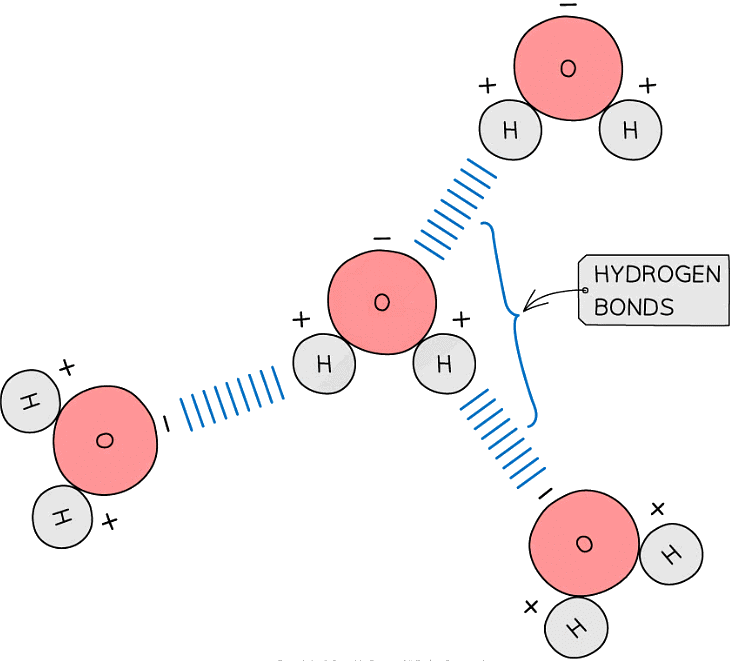
The polarity of water molecules allows hydrogen bonds to form between adjacent water molecules
- Water has many essential roles in living organisms due to its properties:
- The polarity of water molecules
- The presence and number of hydrogen bonds between water molecules
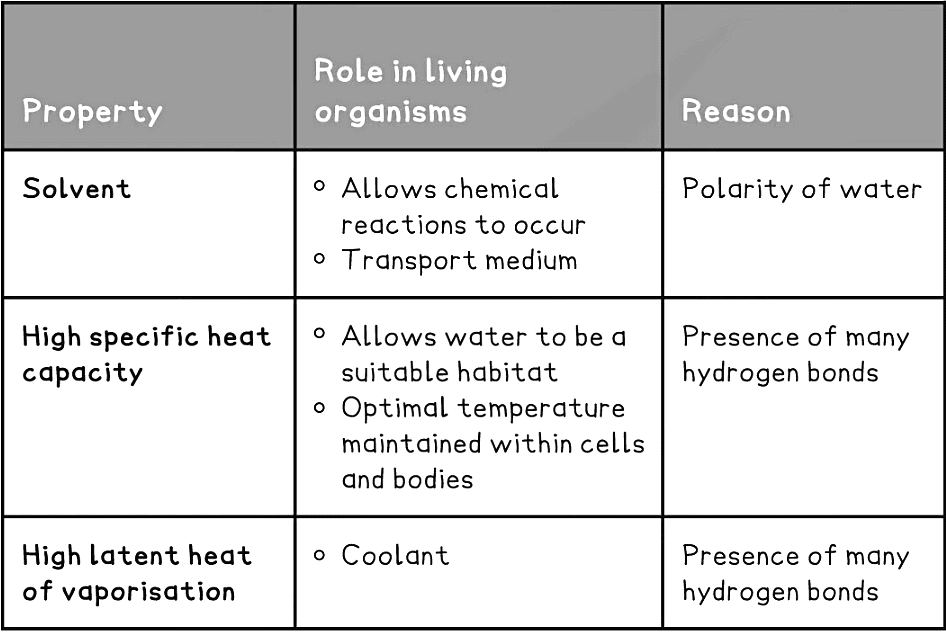
Solvent
- As water is a polar molecule many ions (e.g. sodium chloride) and covalently bonded polar substances (e.g. glucose) will dissolve in it
- This allows chemical reactions to occur within cells (as the dissolved solutes are more chemically reactive when they are free to move about)
- Metabolites can be transported efficiently (except non-polar molecules which are hydrophobic)
Due to its polarity water is considered a universal solvent
High specific heat capacity
- The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°C. Water’s specific heat capacity is 4200 J/kg°C
- Specific heat capacity is a measure of the energy required to raise the temperature of 1 kg of a substance by 1oC
- Water has a high specific heat capacity of 4200 J / Kg oC meaning a relatively large amount of energy is required to raise its temperature
- The high specific heat capacity is due to the many hydrogen bonds present in water. It takes a lot of thermal energy to break these bonds and a lot of energy to build them, thus the temperature of water does not fluctuate greatly
- The advantage for living organisms is that it:
- Provides suitable habitats
- Is able to maintain a constant temperature as water is able to absorb a lot of heat without big temperature fluctuations
- This is vital in maintaining temperatures that are optimal for enzyme activity
- Water in blood plasma is also vital in transferring heat around the body, helping to maintain a fairly constant temperature
- As blood passes through more active (‘warmer’) regions of the body, heat energy is absorbed but the temperature remains fairly constant
- Water in tissue fluid also plays an important regulatory role in maintaining a constant body temperature
Latent heat of vaporisation
- In order to change state (from liquid to gas) a large amount of thermal energy must be absorbed by water to break the hydrogen bonds and evaporate
- This is an advantage for living organisms as only a little water is required to evaporate for the organism to lose a great amount of heat
- This provides a cooling effect for living organisms, for example the transpiration from leaves or evaporation of water in sweat on the skin
Properties of Water & its Role in Living Organisms Table
 Cohesion and adhesion
Cohesion and adhesion
- Hydrogen bonds between water molecules allows for strong cohesion between water molecules
- This allows columns of water to move through the xylem of plants and through blood vessels in animals
- This also enables surface tension where a body of water meets the air, these hydrogen bonds occur between the top layer of water molecules to create a sort of film on the body of water (this is what allows insects such as pond skaters to float)
- Water is also able to hydrogen bond to other molecules, such as cellulose, which is known as adhesion
- This also enables water to move up the xylem due to transpiration
Inorganic Ions
- An ion is an atom (or sometimes a group of atoms) that has an electrical charge
- An ion that has a +ve charge is known as a cation
- An ion that has a -ve charge is known as an anion
- An inorganic ion is an ion that does not contain carbon
- Inorganic ions play an important role in many essential cellular processes
- Inorganic ions occur in solution in the cytoplasm and body fluids of organisms
- Some occur in high concentrations and others in very low concentrations
- The concentration of certain ions can fluctuate and can be used in cell signalling and neuronal transmission
- Each type of inorganic ion has a specific role, depending on its properties
Properties & Roles of Inorganic Ions
- You should know the following inorganic ions, as well as their properties and roles in the body:
- Hydrogen ions (H+)
- Iron ions (Fe2+/Fe3+)
- Sodium ions (Na+)
- Phosphate ions (PO43-)
- Calcium ions (Ca2+)
Hydrogen ions
- Hydrogen ions are protons
- The concentration of H+ in a solution determines the pH
- There is an inverse relationship between the pH value and the hydrogen ion concentration
- The more H+ ions present, the lower the pH (the more acidic the solution)
- The fewer H+ ions present, the higher the pH (the more alkaline the solution)
- The concentration of H+ is therefore very important for enzyme-controlled reactions, which are all affected by pH
- The fluids in the body normally have a pH value of approximately 7.4
- The maintenance of this normal pH is essential for many of the metabolic processes that take place within cells
- Changes in pH can affect enzyme structure
- For example, abnormal levels of hydrogen ions can interact with the side-chains of amino acids and change the secondary and tertiary structures of the proteins that make up enzymes
- This can cause denaturation of enzymes
Iron ions
- There are actually two versions of iron ions (known as oxidation states)
- Iron (II) ions, also known as ferrous ions (Fe2+)
- Iron (III) ions, also known as ferric ions (Fe3+)
- Iron ions are essential as they can bind oxygen
- Haemoglobin is the large protein in red blood cells that is responsible for transporting oxygen around the body
- Haemoglobin is made up of four polypeptide chains that each contain one Fe2+
- This Fe2+ is a key component in haemoglobin as it binds to oxygen
- Myoglobin in muscles functions in a similar way (it is an oxygen-binding protein) but is only made up of one polypeptide chain (containing one Fe2+)
- Iron ions are also essential as they are involved in the transfer of electrons during respiration and photosynthesis, so they are key to the biological generation of energy
- Iron ions are an essential component of cytochromes (that are themselves a component of electron transport chains)
- Cytochrome c contains an iron ion that is essential to its function
- During the electron transport process, this iron ion switches between the Fe3+ and Fe2+ oxidation states, which allows for electrons to be accepted and donated
Sodium Ions
- Na+ is required for the transport of glucose and amino acids across cell-surface membranes (e.g. in the small intestine)
- Glucose and amino acid molecules can only enter cells (through carrier proteins) alongside Na+
- This process is known as co-transport
- First, Na+ is actively transported out of the epithelial cells that line the villi
- The Na+ concentration inside the epithelial cells is now lower than the Na+ concentration in the lumen of the small intestine
- Na+ now re-enters the cells (moving down the concentration gradient) through co-transport proteins on the surface membrane of the epithelial cells, allowing glucose and amino acids to enter at the same time
- Na+ is also required for the transmission of nerve impulses
- PO43- attaches to other molecules to form phosphate groups, which are an essential component of DNA, RNA and ATP
- In DNA and RNA, the phosphate groups allow individual nucleotides to join up (to form polynucleotides)
- In ATP, the bonds between phosphate groups store energy
- These phosphate groups can be easily attached or detached
- When the bonds between phosphate groups are broken, they release a large amount of energy, which can be used for cellular processes
- Phosphates are also found in phospholipids, which are key components of the phospholipid bilayer of cell membranes
Calcium Ions
- Ca2+ is essential in the movement of organisms:
- In synapses, calcium ions regulate the transmission of impulses from neurone to neurone
- Ca2+ also stimulates muscle contraction
- When an impulse reaches a muscle fibre, Ca2+ is released from the sarcoplasmic reticulum
- This Ca2+ binds to troponin C, removing the tropomyosin from myosin-binding sites on actin
- This allows actin-myosin cross-bridges to form when the muscle fibre contracts
- Ca2+ can also help to regulate protein channels, which affects the permeability of cell membranes
- Many enzymes are activated by Ca2+, making these ions key regulators in many biological reactions
- The presence of Ca2+ is also necessary for the formation of blood clots (it is known as a clotting factor)
The document The Structure of ATP | Biology for Grade 12 is a part of the Grade 12 Course Biology for Grade 12.
All you need of Grade 12 at this link: Grade 12
|
109 videos|127 docs|120 tests
|
Related Searches




















