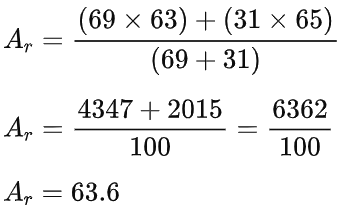Atomic structure | Chemistry for Grade 10 PDF Download
Introduction
- Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The number of subatomic particles in an atom can be calculated from the atom's atomic number and mass number.
Early ideas about atoms
- Ideas about atoms have changed over time. Scientists developed new atomic models as they gathered new experimental evidence.
- John Dalton published his ideas about atoms in 1803. He thought that all matter was made of tiny particles called atoms, which he imagined as tiny spheres that could not be divided.
- Nearly 100 years later, J J Thomson carried out experiments and discovered the electron. This led him to suggest the plum pudding model of the atom. In this model, the atom is a ball of positive charge with negative electrons embedded in it - like currants in a Christmas pudding.
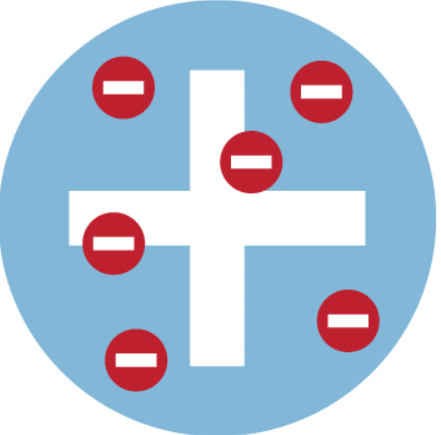
The plum pudding model
- In 1909 Ernest Rutherford designed an experiment to test the plum pudding model. In the experiment, positively charged alpha particles were fired at thin gold foil. Most alpha particles went straight through the foil. But a few were scattered in different directions.
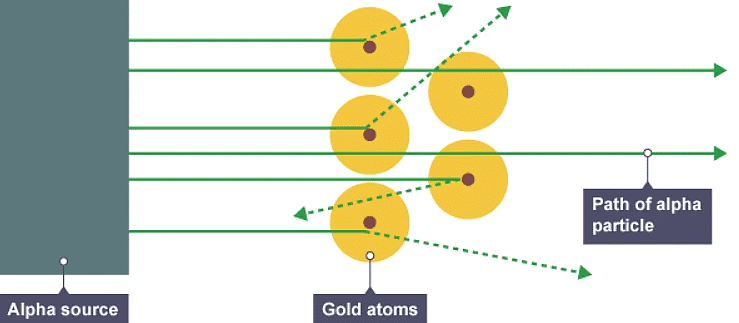
The alpha particle scattering experiment
This evidence led Rutherford to suggest a new model for the atom, called the nuclear model. In the nuclear model:
- the mass of an atom is concentrated at its centre, the nucleus
- the nucleus is positively charged
Developing models of atoms
- Niels Bohr adapted Ernest Rutherford's nuclear model. Bohr did calculations that led him to suggest that electrons orbit the nucleus in shells. The shells are at certain distances from the nucleus. The calculations agreed with observations from experiments.
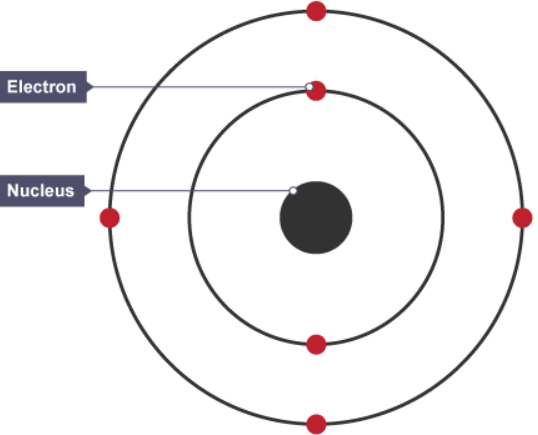
The nuclear model of the atom, showing electrons in shells
- Further experiments led to the idea that the nucleus contained small particles, called protons. Each proton has a small amount of positive charge.
- In 1932 James Chadwick found evidence for the existence of particles in the nucleus with mass but no charge. These particles are called neutrons. This led to another development of the atomic model, which is still used today.
Structure of the atom
Nucleus and shells
- An atom has a central nucleus. This is surrounded by electrons arranged in shells.
- The nucleus is tiny compared to the atom as a whole:
- the radius of an atom is about 0.1 nm (1 × 10-10 m)
- the radius of a nucleus (1 × 10-14 m) is less than 1/10,000 of the radius of an atom
- For comparison, the radius of a typical bacterium is 1 × 10-6 m and the radius of a human hair is about 1 × 10-4 m.
Subatomic particles
- The nuclei of all atoms contain subatomic particles called protons. The nuclei of most atoms also contain neutrons.
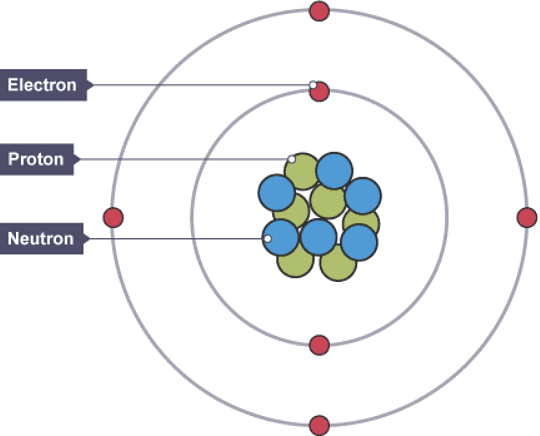
The structure of a carbon atom, not drawn to scale
- The masses of subatomic particles are very tiny. Instead of writing their actual masses in kilograms, we often use their relative masses. The relative mass of a proton is 1, and a particle with a relative mass smaller than 1 has less mass.
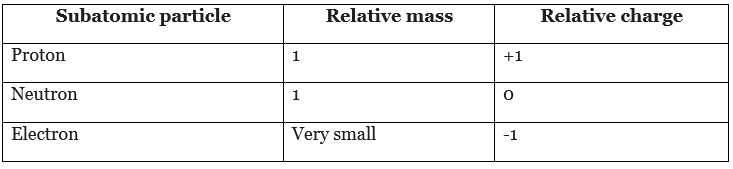
- The mass of an electron is very small compared to a proton or a neutron. Since the nucleus contains protons and neutrons, most of the mass of an atom is concentrated in its nucleus.
- Protons and electrons have electrical charges that are equal and opposite.
Atomic number and mass number
Atomic number
- The number of protons in an atom of an element is its atomic number. Remember that:
- all atoms of a given element have the same number of protons
- atoms of different elements have different numbers of protons
- An atom contains equal numbers of protons and electrons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.
- For example, the atomic number of sodium is 11. Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges.
Mass number
The mass number of an atom is its total number of protons and neutrons.
- Atoms of different elements usually have different mass numbers, but they can be the same. For example, the mass number of argon atoms and calcium atoms can both be 40.
Calculating numbers of subatomic particles
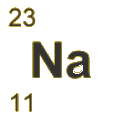
- The symbol for an atom can be written to show its mass number at the top, and its atomic number at the bottom.
- To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number:
- number of protons = atomic number
- number of electrons = atomic number
- number of neutrons = mass number - atomic number
Example: The atomic number of a sodium atom is 11 and its mass number is 23. Calculate the number of protons, neutrons and electrons it contains.
Number of protons = 11
Number of electrons = 11
Number of neutrons (mass number - atomic number) = 23 - 11 = 12
Isotopes
- Atoms of the same element must have the same number of protons, but they can have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes of an element have:
- the same atomic number
- different mass numbers
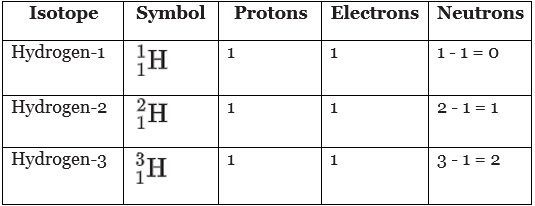
Three isotopes of hydrogen
- All hydrogen atoms contain one proton (and one electron), but they can contain different numbers of neutrons. Hydrogen-1 is the most abundant (most common) isotope of hydrogen.
- An isotope is named after the element and the mass number of its atoms. For example, carbon-12 is an isotope of carbon with a mass number of 12.
- All three isotopes of hydrogen have identical chemical properties. This is because the number of electrons determines chemical properties, and all three isotopes have one electron in their atoms.
Relative atomic mass
The relative atomic mass of an element is a weighted average of the masses of the atoms of the isotopes. It takes account of the abundance of each of the isotopes of the element.
Relative atomic masses can be found in the periodic table. They have the symbol Ar.
Take care not to confuse mass numbers and relative atomic masses:
- mass numbers are always whole numbers (protons or neutrons cannot be split into parts)
- relative atomic masses are often rounded to the nearest whole number, but are actually not whole numbers
For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. This is because chlorine contains two different isotopes, chlorine-35 and chlorine-37.
Calculating relative atomic mass
- The carbon-12 atom,
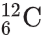 is the standard atom against which the masses of other atoms are compared. The relative atomic mass of an element is the average mass of its atoms, compared to 1/12th the mass of a carbon-12 atom. The relative atomic mass, Ar, of an element is calculated from:
is the standard atom against which the masses of other atoms are compared. The relative atomic mass of an element is the average mass of its atoms, compared to 1/12th the mass of a carbon-12 atom. The relative atomic mass, Ar, of an element is calculated from:- the mass numbers of its isotopes
- the abundance of these isotopes
Chlorine
- Chlorine naturally exists as two isotopes,
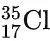 (chlorine-35) and
(chlorine-35) and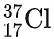 (chlorine-37). The abundance of chlorine-35 is 75% and the abundance of chlorine-37 is 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
(chlorine-37). The abundance of chlorine-35 is 75% and the abundance of chlorine-37 is 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37. - To calculate the relative atomic mass, Ar, of chlorine:
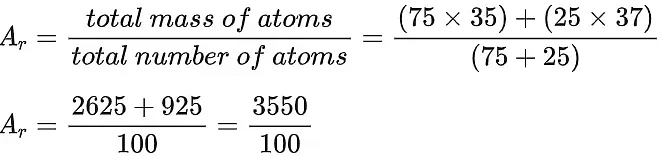
Ar = 35.5 (to 1 decimal place) - Notice that the answer is closer to 35 than it is to 37. This is because the chlorine-35 isotope is much more abundant than the chlorine-37 isotope.
Example: The table shows the mass numbers and abundances of naturally occurring copper isotopes.

Calculate the relative atomic mass of copper. Give your answer to 1 decimal place.
|
77 videos|200 docs|26 tests
|