Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Using Moles to Balance Equations
Using Moles to Balance Equations | Chemistry for Grade 10 PDF Download
Using Moles to Balance Equations
Higher Tier Only- Stoichiometry refers to the numbers in front of the reactants and products in an equation, which must be adjusted to make sure that the equation is balanced
- These numbers are called coefficients (or multipliers) and if we know the masses of reactants and products, the balanced chemical equation for a given reaction can be found by determining the coefficients
- First, convert the masses of each reactant and product in to moles by dividing by the molar masses using the periodic table
- If the result yields uneven numbers, then multiply all of the numbers by the same number, to find the smallest whole number for the coefficient of each species
- For example, if the resulting numbers initially were 1, 2 and 2.5, then you would multiply all of the numbers by 2, to give the whole numbers 2, 4 and 5
- Then, use the molar ratio to write out the balanced equation
Solved Example
64 g of methanol, CH3OH, reacts with 96 g of oxygen gas to produce 88 g of carbon dioxide and 72 g of water. Deduce the balanced equation for the reaction. (C = 12, H = 1, O = 16)
- Calculate the molar masses of the substances in the equation
CH3OH = 32 g / mol O2 = 32 g / mol
CO2 = 44 g / mol H2O = 18 g / mol- Divide the masses present by the molar mass to obtain the number of moles
CH3OH = 64 g ÷ 32 g mol-1 = 2 mol
O2 = 96 g ÷ 32 g mol-1 = 3 mol
CO2 = 88 g ÷ 44 g mol-1 = 2 mol
H2O = 72 g ÷ 18 gmol-1 = 4 mol- The mole ratios are the same as the coefficients in the balanced equation
2CH3OH + 3O2 ⟶ 2CO2 + 4H2O
Exam Tip
The molar ratio of a balanced equation gives you the ratio of the amounts of each substance in the reaction.
- For the reaction of elements forming compounds, it can be necessary to determine the formula of one of the products in order to balance the equation
- This involves calculating the empirical formula
- Write each element involved
- Determine the mass of each element
- Write the atomic mass of each element
- Calculate the number of moles of each element (divide by the atomic mass)
- Calculate the ratio of elements (divide by the smallest answer)
- Write the final empirical formula
- With the formula of the product, it is then possible to write a balanced symbol equation
Exam Tip
- The empirical formula is the simplest whole number ratio of all elements in a substance
- If the value that you calculate is very close to a whole number, then you can round it off
- e.g. 1.003 ≈ 1
- If the value that you calculate is not close to a whole number, then you might need to double or triple all of the values you have
- 0.3324 and 1.003 would become 0.3324 x 3 ≈ 1 and 1.003 x 3 ≈ 3
Solved Example
10 g of hydrogen and 80 g of oxygen react to form one product. Deduce the balanced equation for the reaction. (C = 12, H = 1, O = 16)
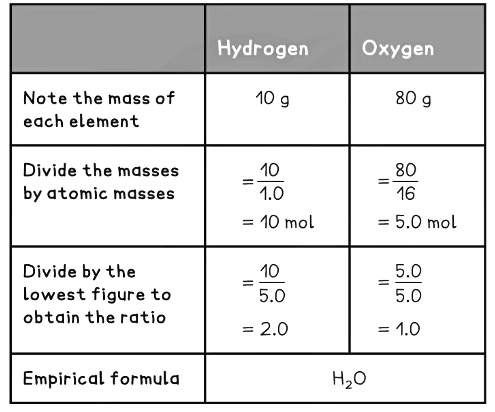
- The equation can then be balanced as normal
- ___H2 + ___O2 → ___H2O
The O2 suggests that 2H2O is needed - ___H2 + ___O2 → 2H2O
The 2H2O suggests that 2H2 is needed - 2H2 + O2 → 2H2O
- The equation is balanced
- The reactants could be balanced using the technique shown in the previous worked example
- However, there is not sufficient information to balance the products
- 10 moles of hydrogen and 5 moles of oxygen is 2 : 1
- But there is no information about the product
- 2H2 + O2 → H2O
- The equation is unbalanced
Exam Tip
At GCSE level, it is unlikely that you will be given the information in percentages but if you are just treat them as though the percentage value is the mass
The document Using Moles to Balance Equations | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
78 videos|87 docs|11 tests
|
Related Searches















