Grade 10 Exam > Grade 10 Notes > Physics for Grade 10 > Radioactive Decay
Radioactive Decay | Physics for Grade 10 PDF Download
| Table of contents |

|
| Unstable Nuclei |

|
| Radiation |

|
| Tips |

|
| Activity |

|
| Detecting Radiation |

|
Unstable Nuclei
- Some atomic nuclei are unstable
- This is because of an imbalance in the forces within the nucleus
- Forces exist between the particles in the nucleus
- Carbon-14 is an isotope of carbon which is unstable
- It has two extra neutrons compared to stable carbon-12
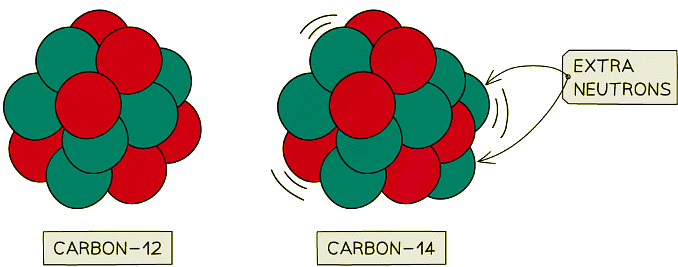 Carbon-12 is stable, whereas carbon-14 is unstable. This is because carbon-14 has two extra neutrons
Carbon-12 is stable, whereas carbon-14 is unstable. This is because carbon-14 has two extra neutrons - Some isotopes are unstable because of their large size or because they have too many or too few neutrons
- It has two extra neutrons compared to stable carbon-12
Radiation
- Unstable nuclei can emit radiation to become more stable
- Radiation can be in the form of a high energy particle or wave
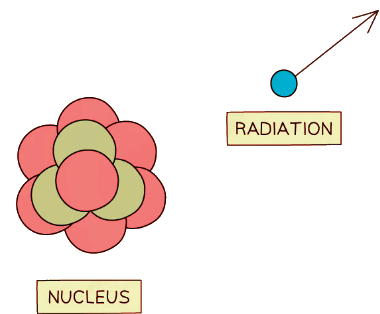 Unstable nuclei decay by emitting high energy particles or waves
Unstable nuclei decay by emitting high energy particles or waves - As the radiation moves away from the nucleus, it takes some energy with it
- This reduces the overall energy of the nucleus
- This makes the nucleus more stable
- The process of emitting radiation is called radioactive decay
- Radioactive decay is a random process
- This means it is not possible to know exactly when a particular nucleus will decay
Tips
- The terms unstable, random and decay have very particular meanings in this topic. Remember to use them correctly when answering questions!
- Do not confuse activity and count rate. Activity is the rate at which unstable nuclei decay, whereas count rate is the rate at which radioactive emissions are detected.
 |
Download the notes
Radioactive Decay
|
Download as PDF |
Download as PDF
Activity
- Objects containing radioactive nuclei are called sources of radiation
- Sources of radiation decay at different rates which are defined by their activity
- The activity is defined as
- The rate at which the unstable nuclei from a source of radiation decays
- Activity is measured in Becquerels
- The symbol for Becquerels is Bq
- 1 Becquerel is equal to 1 nucleus in the source decaying in 1 second
Example: A source of radiation has an activity of 2000 Bq. How many unstable atoms decay in 2 minutes?
Step 1: Determine the activity
- The activity of the source is 2000 Bq
- This means 2000 nuclei decay every second
Step 2: Determine the time period in seconds
- The time period is 2 minutes
- Each minute has 60 seconds
- The time period in seconds is:
2 × 60 = 120 secondsStep 3: Multiply the activity by the time period
Activity (Bq) × Time period (s) = 2000 × 120 = 240 000
Therefore, 240 000 unstable nuclei decay in 2 minutes
Detecting Radiation
- Radiation that is emitted from an unstable nucleus can be detected in different ways
- For example, photographic film changes colour when exposed to radiation
- A Geiger-Muller tube is a device used to detect radiation
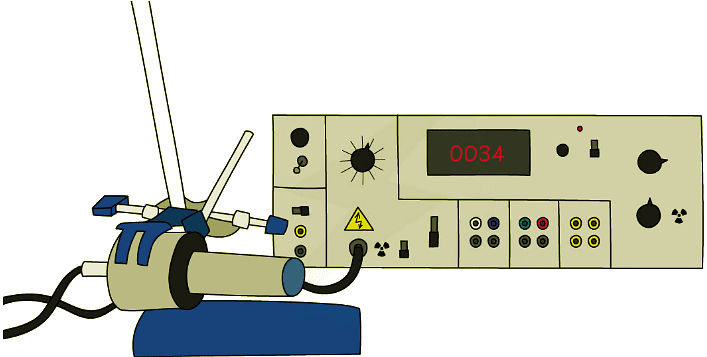 This Geiger-Muller Tube is connected to a Geiger Counter. This a common way of detecting radiation and measuring a count-rate
This Geiger-Muller Tube is connected to a Geiger Counter. This a common way of detecting radiation and measuring a count-rate - Within the Geiger-Muller tube, ions are created by radiation passing through it
- The Geiger-Muller tube can be connected to a Geiger counter
- This counts the ions created in the Geiger-Muller tube
- Count-rate is the number of decays recorded each second by a detector
Example: A Geiger-Muller tube is used to detect radiation in a particular location. If it counts 16,000 decays in 1 hour, what is the count rate?
Step 1: Identify the different variables
- The number of decays is 16 000
- The time is 1 hour
Step 2: Determine the time period in seconds
- 1 hour is equal to 60 minutes, and 1 minute is equal to 60 seconds
Time period = 1 × 60 × 60 = 3600 secondsStep 3: Divide the total counts by the time period in seconds
Counts ÷ Time period = 16 000 ÷ 3600 = 4.5
Therefore, there are 4.5 decays per second
The document Radioactive Decay | Physics for Grade 10 is a part of the Grade 10 Course Physics for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
124 videos|149 docs|37 tests
|
Related Searches















