Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Calculating Rates of Reactions
Calculating Rates of Reactions | Chemistry for Grade 10 PDF Download
How to Calculate Rates of Reactions?
- Reactions take place at different rates depending on the identities and conditions
- Some are extremely slow e.g. rusting and others are extremely fast e.g. explosives
- Rates of reaction can be measured either by how fast a reactant is used up or by how fast the product is made
- Rate is concerned with amounts of substances and time and can be calculated using the formula:
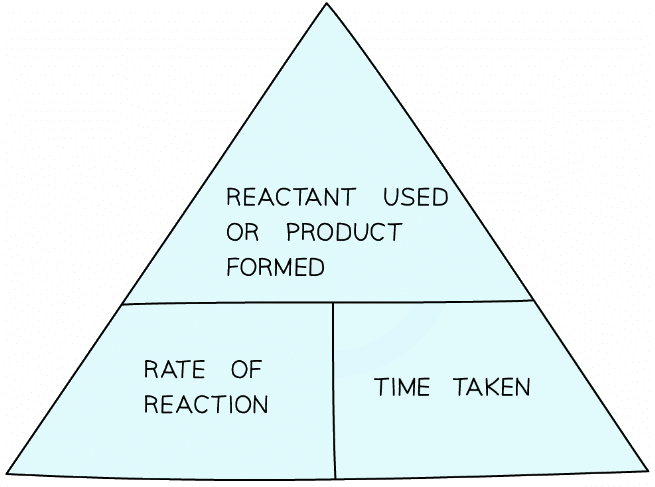 A formula triangle for calculating the rate of reaction
A formula triangle for calculating the rate of reaction
- In order to provide sufficient data to establish a conclusion several measurements need to be made during the reaction
- The product is usually the one that is measured as it is usually easier to measure a product forming than it is a reactant disappearing
- The quantity to be measured depends on the reaction and may be in grams for mass or cm3 or dm3 for volume if the product is a gas
- The units of the rate of reaction would therefore be g/s or cm3/s or dm3/s
- Time is usually in seconds as many reactions studied in the lab are quite quick
- If one of the products is a gas which is given off, then the reaction can be performed in an open flask on a balance to measure the loss in mass of reactant
- Cotton wool is usually placed in the mouth of the flask which allows gas out but prevents any materials from being ejected from the flask (if the reaction is vigorous)
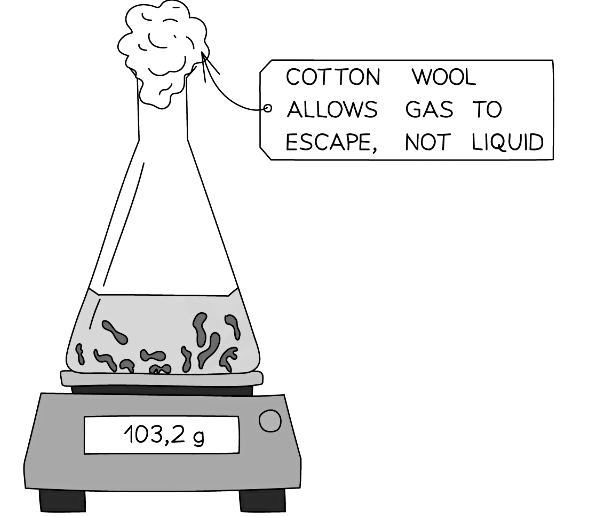 Diagram showing the set-up for measuring the rate of reaction by loss in mass
Diagram showing the set-up for measuring the rate of reaction by loss in mass
- This method is not suitable for hydrogen and other gases with a small relative formula mass, Mr as the loss in mass may be too small to measure
- Alternatively, the gas could be captured in a gas syringe which measures its volume
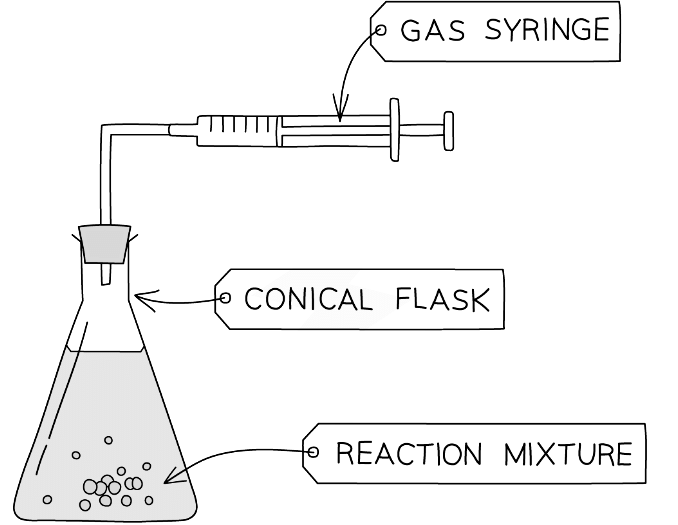 Diagram of a gas syringe used to determine the rate of reaction
Diagram of a gas syringe used to determine the rate of reaction
- Precipitation reactions form a solid precipitate when two clear solutions are mixed together
The precipitate clouds the reaction mixture so if the flask is placed over a piece of paper with a cross on it, the time it takes for the cross to disappear from view (due to the formation of the precipitate) can be measured
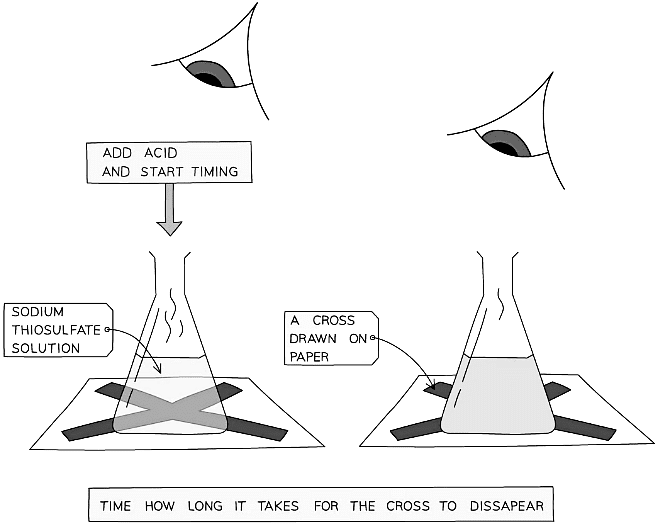 Diagram showing the study of a rate of precipitation
Diagram showing the study of a rate of precipitation- This method is susceptible to error though as they are subjective, given that different people may not agree on the exact moment that the cross disappears
- Another disadvantage is that only one data point is produced per experiment, so a rate of reaction graph cannot be plotted
Exam Tip
When answering questions on the effect of concentration/pressure on the rate of reaction, you should mention that there are more particles per unit volume (usually cm3) rather than just more particles.
The document Calculating Rates of Reactions | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches















