Tests for Gases | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Test for Hydrogen |

|
| Test for Oxygen |

|
| Test for Carbon Dioxide |

|
| Test for Chlorine |

|
Test for Hydrogen
Squeaky Pop Test
- The test for hydrogen consists of holding a burning splint held at the open end of a test tube of gas
- If the gas is hydrogen it burns with a loud “squeaky pop” which is the result of the rapid combustion of hydrogen with oxygen to produce water
- Be sure not to insert the splint right into the tube, just at the mouth, as the gas needs air to burn
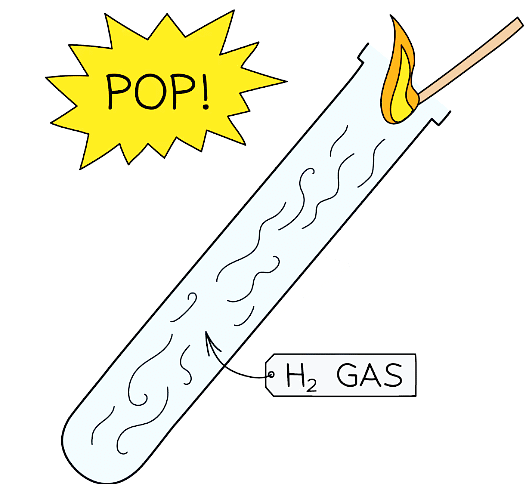 Diagram showing the test for hydrogen gas
Diagram showing the test for hydrogen gas
Exam Tip
It is easy to confuse the tests for hydrogen and oxygen. Try to remember that a ligHted splint has an H for Hydrogen, while a glOwing splint has an O for Oxygen.
Test for Oxygen
Glowing Splint Test
- The test for oxygen consists of placing a glowing splint inside a test tube of gas
- If the gas is oxygen the splint will relight
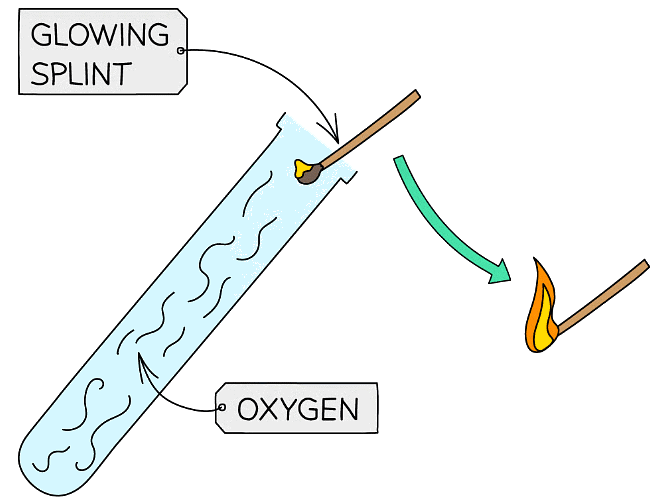 Diagram showing the test for oxygen gas
Diagram showing the test for oxygen gas
Exam Tip
Sometimes the splint does not relight, but it glows very brightly, which is also a positive result. In an exam, however, it is best to state it relights the glowing splint.
Test for Carbon Dioxide
Limewater Test
- The test for carbon dioxide involves bubbling the gas through an aqueous solution of calcium hydroxide (limewater)
- If the gas is carbon dioxide, the limewater turns milky or cloudy
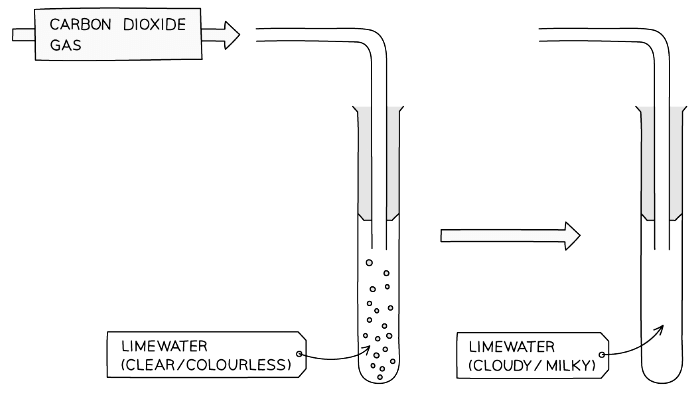 Diagram showing the test for carbon dioxide gas
Diagram showing the test for carbon dioxide gas
Exam Tip
Sometimes students think that extinguishing a burning splint indicates carbon dioxide gas. However, while it is a property of carbon dioxide, other gases, such as nitrogen, will also do this, so the test is not definitive and should not be quoted in an exam answer.
Test for Chlorine
Litmus Paper Test
- The test for chlorine makes use of litmus paper
- If chlorine gas is present, damp blue litmus paper will be bleached white
- It may turn red briefly before bleaching, as acids are produced when chlorine comes into contact with water
- Chlorine should always be handled in a fume cupboard due to its toxicity
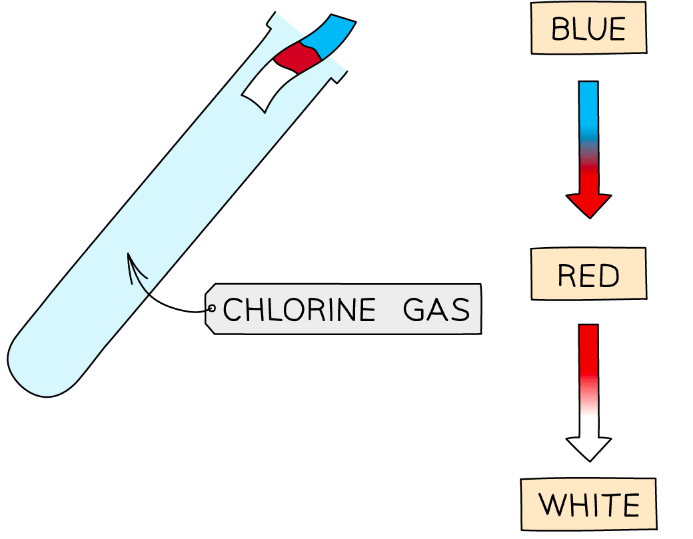 Diagram showing the test for chlorine gas
Diagram showing the test for chlorine gas
Exam Tip
You should distinguish between properties of gases and tests for gases. Chlorine 'smells like swimming pools' is a characteristic, but not an acceptable means of identification. You can use blue, red or universal indicator paper to show the bleaching effect.
|
75 videos|131 docs|24 tests
|















