Enzyme Structure and Function | Biochemistry for MCAT PDF Download
| Table of contents |

|
| Enzymes: what they are and what they do |

|
| The definition of catalysis |

|
| How do catalysts work? |

|
| Why enzymes are so important |

|
Enzymes: what they are and what they do
- Inside our bodies, billions of chemical reactions occur rapidly within cells, thanks to the presence of enzymes. These reactions, which occur at lightning speed, are essential for various biochemical processes. However, when these same reactions are attempted in a laboratory setting, they often proceed at a significantly slower pace. The discrepancy in reaction rates can be attributed to the remarkable abilities of enzymes.
- Enzymes act as catalysts, facilitating and accelerating chemical reactions. Their presence within living organisms creates conditions that promote faster reaction rates, ensuring the efficient functioning of biological processes. In contrast, a test tube lacks the complex and specific properties of enzymes that enable them to catalyze reactions effectively, resulting in a slower pace of chemical reactions outside of a living system.
The definition of catalysis
Let's examine a chemical reaction involving the bonding of molecule A with molecule B, resulting in the formation of molecule A-B, where A is attached to B. The rate at which this reaction occurs is determined by specific conditions such as temperature, atmospheric pressure, and the concentrations of the reactants A and B, as well as the product A-B. For instance, in a given scenario, it might be observed that three molecules of A combine with three molecules of B, resulting in the production of three molecules of A-B per second.

For a substance to be classified as a catalyst in this reaction, it needs to fulfill two requirements. Firstly, it must enhance the rate of the reaction, such as increasing the production of A-B molecules from 3 per second to 16 per second, as an example. Secondly, the catalyst should not be consumed or permanently altered by the reaction. It must remain intact and available even after the reaction has completed. In essence, a catalyst accelerates a reaction without undergoing any permanent changes itself.
The latter aspect of the catalyst definition holds significant significance. To illustrate, let's consider starting a campfire, which involves a chemical reaction between wood and oxygen. If we were to expedite the reaction by pouring a substantial bucket of gasoline onto the fire, it would certainly accelerate the process. However, this would not qualify as catalyzing the fire reaction. Although the gasoline enhances the reaction, as evidenced by the unfortunate consequences of burnt eyebrows and singed hair for anyone attempting this experiment, it is also completely consumed during the process. Consequently, there is no gasoline remaining once the fire is extinguished. Hence, gasoline used in this manner is not considered a catalyst.
If gasoline isn’t a catalyst, what is?
An excellent everyday illustration of a catalyst can be found in the emissions control system of your car, prominently featuring a component known as a catalytic converter. This converter is designed as a container comprising a series of small screens coated with precious metals like platinum, rhodium, and others. These metals act as catalysts in the conversion of nitric oxide, which consists of a nitrogen atom bonded to an oxygen atom, into separate nitrogen and oxygen molecules. The presence of these metals within the catalytic converter expedites the transformation of nitric oxide, resulting in the production of nitrogen and oxygen. Importantly, unlike the gasoline used on a fire, these metals are not depleted or consumed during the process. Therefore, in theory, a catalytic converter should continue to function efficiently long after other parts of your car have worn out or ceased to work.
How do catalysts work?
- The fundamental operation of most catalysts, including enzymes, follows a similar mechanism due to the shared characteristics of many chemical reactions, including biochemical processes.
- To illustrate this principle, let's examine the nitric oxide reaction discussed earlier. In this case, the reaction involves the collision between two nitric oxide molecules, leading to the rupture of nitrogen-oxygen bonds and the formation of new nitrogen-nitrogen and oxygen-oxygen bonds. This example serves as a concise demonstration of how catalysts function, where they facilitate the rearrangement of chemical bonds during the collision of reactant molecules.

If we were to introduce a large quantity of nitric oxide molecules into a regular jar without a catalytic converter and observe the molecular-level interactions up close, we would witness a chaotic scene. Millions of N-O molecules would be in constant motion, spinning, tumbling, and colliding with one another and the walls of the jar at astonishing velocities. However, the vast majority of these collisions would not lead to any chemical reactions. Only a very small number of nitrogen or oxygen molecules would be formed, while the majority of the nitric oxide molecules would simply rebound off each other. The question arises: Why does this occur?
Why the nitric oxide molecules bounce off each other: a thought experiment
- To visualize a molecule such as nitric oxide, we can imagine it as two magnets joined together. When playing with magnets, you may have noticed that to make them stick, you need to align the opposite poles of each magnet: north to south or south to north. Conversely, if you attempt to align the same poles together, the magnets will repel each other. Nitrogen and oxygen atoms exhibit similar behavior to magnets in this regard. When aligned in a precise manner, with the "north pole" of nitrogen positioned next to the "south pole" of oxygen, they form a bonded molecule known as nitric oxide. In essence, chemical "bonds" arise from attractive forces between atoms, similar to the magnetic attraction between the poles of magnets.

- Let's simplify the concept by envisioning oxygen atoms as red magnets and nitrogen atoms as blue magnets. We can establish two rules regarding the behavior of these magnets. The first rule states that there is a mutual attraction between red magnets and blue magnets. This means that if you bring the north pole of a red magnet close to the south pole of a blue magnet, they will stick together, similar to the behavior of magnets. The second rule dictates that there is a stronger attraction between magnets of the same color, meaning red magnets prefer to stick to other red magnets, and blue magnets prefer to stick to other blue magnets when given the choice.
- Now, let's consider what occurs when a red magnet attached to a blue magnet collides with another red magnet attached to a blue magnet. If the poles of the colliding magnets are aligned correctly, with the north pole of one red magnet contacting the south pole of the other red magnet, and the same alignment occurring for the blue magnets, an interesting outcome arises. Due to the stronger attraction between magnets of the same color, the collision results in the "destruction" of the red-blue "molecules" and the formation of one "red-red" molecule and one "blue-blue" molecule. However, it is crucial for the alignment to be precise. If the red-blue molecules do not align correctly, they will simply bounce off each other, and no new molecules will be created.

- The magnet thought experiment provides a useful approximation of the behavior of real-life molecules like nitric oxide. When two N-O molecules collide in the exact right orientation, with nitrogen atoms aligned with nitrogen atoms and oxygen atoms aligned with oxygen atoms, the original N-O molecules will transform into new N-N and O-O molecules. However, it is crucial to emphasize that this alignment is essential for the reaction to occur; without it, nothing will happen.
- This is precisely where catalysts play a crucial role. Catalysts assist in achieving the necessary alignment. To better understand this, let's return to the magnet analogy. Imagine a jar filled with numerous small red-blue magnet "molecules," and let's shake the jar vigorously, causing the magnets to collide randomly with one another. Occasionally, two red-blue magnets will collide in the correct alignment, resulting in the creation of a red-red and a blue-blue magnet pair. However, there are significantly more incorrect alignments than correct ones, meaning that colliding red-blue magnets are more likely to bounce off each other than to form new "molecules." The odds are generally stacked against any significant changes occurring.
This is what happens with nitric oxide molecules in a jar, when no catalyst is present.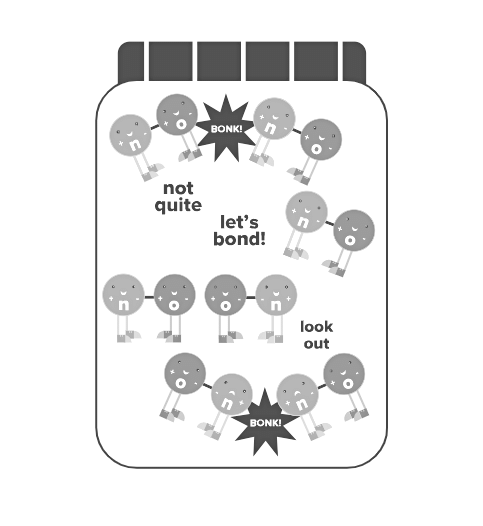 Now, let's consider a scenario where we introduce a remarkably diligent and conscientious magic gnome into the jar. This gnome is given the specific task of grabbing a red-blue magnet pair in each hand, aligning them correctly, and then forcefully bringing them together. With the addition of this helpful gnome assistant, the rate at which red-red and blue-blue magnet pairs are formed will significantly increase. This is because achieving the desired alignment is no longer dependent on random chance alone. We now have an active participant inside the jar, ensuring that the desired alignments are consistently created.
Now, let's consider a scenario where we introduce a remarkably diligent and conscientious magic gnome into the jar. This gnome is given the specific task of grabbing a red-blue magnet pair in each hand, aligning them correctly, and then forcefully bringing them together. With the addition of this helpful gnome assistant, the rate at which red-red and blue-blue magnet pairs are formed will significantly increase. This is because achieving the desired alignment is no longer dependent on random chance alone. We now have an active participant inside the jar, ensuring that the desired alignments are consistently created.
Catalysts serve as the tangible counterparts to our imaginative magic gnomes. Within a catalytic converter, for example, a platinum screen acts as a catalyst by attracting nitric oxide molecules and aligning them in a precise manner. This alignment facilitates successful collisions, allowing the nitrogen and oxygen atoms to rearrange and form nitrogen gas and oxygen gas. Catalysts enhance the speed of reactions by aligning the reactant molecules in such a way that the likelihood of successful reactions is significantly increased.
Enzymes are biological catalysts
Enzymes, the catalysts involved in biological chemical reactions, can be likened to the "gnomes" residing within every living organism. These remarkable entities play a crucial role in aligning molecules, such as nucleotides, to form DNA or combining amino acids to synthesize proteins, among numerous other vital functions. Enzymes are indispensable for life to such an extent that scientists found the term "catalyst" insufficient and introduced the term "enzyme" to emphasize their significance and complexity.Why enzymes are so important
Enzymes play a crucial role in preserving cellular energy, which is a precious resource for living organisms. To understand how enzymes contribute to energy preservation and why it matters, let's revisit our magnet thought experiment.- As we previously observed, adding a magic gnome accelerated the production of red-red and blue-blue "molecules" by ensuring proper alignment during collisions. Alternatively, we could increase the speed by shaking the jar more vigorously. Although this intensified shaking wouldn't directly influence alignment like the gnome does, it would increase the total number of collisions between red-blue magnets per second. By shaking the jar more vigorously, the overall frequency of collisions would rise, thereby increasing the likelihood of correctly aligned collisions occurring as a matter of probability. Consequently, shaking the jar harder, potentially with significantly greater force, would lead to an accelerated production of red-red and blue-blue pairs, similar to the effect of adding a gnome while maintaining the same shaking intensity.
- This analogy illustrates how enzymes contribute to preserving cellular energy. Enzymes facilitate biochemical reactions by accelerating the rate of collisions between molecules involved in the reaction, much like shaking the jar harder increases the frequency of magnet collisions. By increasing the efficiency of collision encounters, enzymes enable the same reaction to occur at a faster rate without requiring additional energy input. This energy-saving aspect is crucial for living organisms as it allows them to perform essential functions while conserving their limited energy resources.

- Let's explore the pros and cons of the two strategies in the magnet thought experiment. Shaking the jar harder, as we mentioned earlier, increases the rate of collisions between red-blue magnets, resulting in a higher production of red-red and blue-blue pairs. The advantage of this approach is that it yields the desired outcome without the need for an additional agent like the gnome. However, it comes with the drawback of requiring more energy expenditure on your part. You have to exert more effort and potentially waste energy in the process.
- On the other hand, using the gnome as a catalyst provides a different set of advantages. By employing the gnome, you can save energy for other purposes. You don't need to shake the jar as vigorously because the gnome takes care of aligning the magnets correctly, leading to the formation of red-red and blue-blue pairs. This energy-saving aspect becomes particularly crucial when energy is scarce or when obtaining energy requires significant work. Additionally, if you have excess energy available, you may prefer to allocate it to other important tasks rather than spending it on shaking the jar harder. In these scenarios, the gnome's assistance and the resulting energy savings can make a significant difference.
- Now, let's consider how this logic applies to living organisms, using the example of a rabbit in a field. The cells in the rabbit's body undergo countless chemical reactions every second, requiring energy to maintain the necessary conditions for these reactions. The rabbit obtains energy by consuming grass, which is converted into simple sugars and further transformed into fuel molecules. Just as shaking the jar releases energy, the burning of fuel molecules within the cells releases energy that accelerates the movement of molecules inside the cells. This increased speed, facilitated by energy expenditure, promotes more collisions and reactions.
- Through millions of years of evolution, the rabbit's cells have been optimized to burn a specific and relatively constant amount of energy. Enzymes play a vital role in this process. With the help of enzymes, the required amount of energy is utilized efficiently. Enzymes ensure that molecules are properly aligned, allowing the desired reactions to occur at the necessary speed without excessive energy consumption. Removing enzymes from the equation would result in significantly slower reaction rates, as the random collisions would take longer to produce the desired outcomes.
- For the rabbit, the speed of chemical reactions in its cells is crucial for its survival. Rapid reactions enable the rabbit to respond quickly to threats or opportunities in its environment. If the reactions were slowed down even slightly, the rabbit's ability to evade predators or obtain food would be compromised. Enzymes provide the optimal solution by allowing fast reactions without incurring unnecessary energy costs.
- Considering the advantages of enzymes, it becomes apparent why they are essential for life. Enzymes enable living organisms to achieve rapid reactions while efficiently managing their limited energy resources. They are the result of a long evolutionary process, starting with the emergence of enzymes in early organisms. These initial advantages conferred by enzymes provided a slight edge in reproduction and survival, leading to the proliferation of organisms with improved enzyme systems. Over millions of years, new enzymes and better-adapted life forms evolved, culminating in complex organisms like the rabbit, capable of reacting swiftly to external stimuli and ensuring their survival.
|
138 videos|21 docs|26 tests
|














