Practice Passage Test - 3 | Practice Passages for MCAT PDF Download
Passage
Lyophilization, also known as freeze drying, is a method employed to eliminate moisture from samples. By utilizing sublimation, lyophilization directly converts frozen water, in its solid state, into water vapor. This approach presents advantages over liquid-based solvent removal techniques because it can be carried out at low temperatures, minimizing potential damage to samples that are sensitive to heat.
To perform lyophilization, a chamber containing the sample solution is connected to a lyophilizer. The lyophilizer first freezes the sample at a temperature below 0°C and then creates a vacuum, maintaining a pressure below 0.006 atm. Under these specific conditions, the frozen water undergoes sublimation, and the resulting water vapor is drawn from the sample chamber into a condenser set at -50°C. In the condenser, the water vapor is refrozen and held stationary.
The accompanying diagram depicts the phase diagram for water, illustrating the distinct negatively inclined equilibrium line between the solid and liquid phases. At low pressures, the solid phase of water is preferred, as long as any solute present in the water remains reasonably diluted. However, as the concentration of solute increases, the freezing point of water decreases. If the sample becomes concentrated enough, a decrease in pressure may cause it to melt rather than sublime. Furthermore, volatile solvents often cannot be eliminated from the chamber through the condenser, as their freezing point at low pressures falls below -50°C. The presence of volatile solvents in the gaseous environment can further contribute to impurities in the remaining water, thereby favoring the liquid phase even more.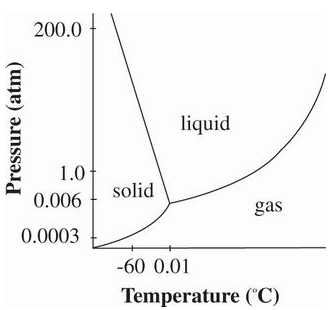 Figure 1 Phase diagram of water
Figure 1 Phase diagram of water















