What is Potential Energy?: Definition, Formula & Curves | AP Physics 1 - Grade 9 PDF Download
Introduction
At its core, potential energy stems from an object's ability to store energy due to its position. For instance, when a bow is drawn, it accumulates potential energy, which transforms into kinetic energy upon release. Similarly, a stretched spring gains potential energy, evident in the tension we feel when stretching it. Therefore, potential energy can be defined as a form of energy resulting from positional or state alterations.
Potential Energy Formula
The formula for potential energy relies on the specific force acting on the objects. In the case of gravitational force, the formula is as follows:
W = m×g×h = mgh
Where:
- m represents the mass of the object in kilograms
- g denotes the acceleration due to gravity
- h signifies the height of the object in meters
Potential Energy Unit
Gravitational potential energy shares the same units as kinetic energy: kg m²/s². It's important to note that all forms of energy possess the same units and are measured in joules (J).
Types of Potential Energy
Potential energy manifests in various forms. The two main types are gravitational potential energy and elastic potential energy.
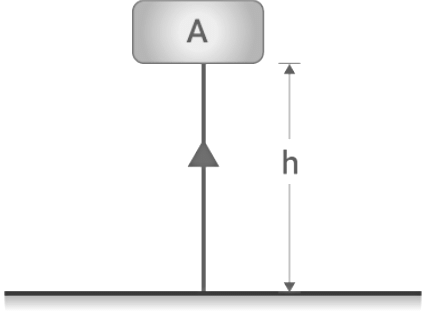
- Gravitational Potential Energy: Gravitational potential energy refers to the energy possessed by an object that has been raised to a certain height against the force of gravity. Let's illustrate this with an example: Imagine an object with mass (m) placed at height (h) above the ground. When the object is raised against gravity, work (W) is done on it. According to the law of conservation of energy, the energy gained by the object, in this case, is its potential energy (E). Thus, the gravitational potential energy (E) of an object raised to a height (h) above the ground is given by the formula: E = m * g * h. Notably, the distance traveled by the object is not considered; only the displacement between the initial and final heights matters.
- Elastic Potential Energy: Elastic potential energy resides in objects that can be compressed or stretched, such as rubber bands, trampolines, or bungee cords. The greater an object's capacity for deformation, the more elastic potential energy it possesses. Some examples of objects designed to store elastic potential energy include a twisted rubber band propelling a toy plane, a stretched bow in archery, or a coiled spring in a wind-up clock. Elastic potential energy can be calculated using the formula: U = 0.5 * k * x², where U represents elastic potential energy, k symbolizes the spring force constant, and x denotes the stretch length in meters.
Potential Energy Surfaces
A potential energy surface (PES) represents the potential energy of a collection of atoms, typically in terms of their spatial coordinates. The PES can describe energy as a function of one or more coordinates, with a potential energy curve or energy profile representing a PES with a single coordinate. The analogy of a landscape is often used, where energy values correspond to different bond lengths or other relevant variables. Each geometry of atoms in a chemical reaction corresponds to a unique potential energy, resulting in a smooth energy "landscape" that allows the study of chemistry from a topological perspective.
Potential Energy Curves: Understanding 1-D Surfaces
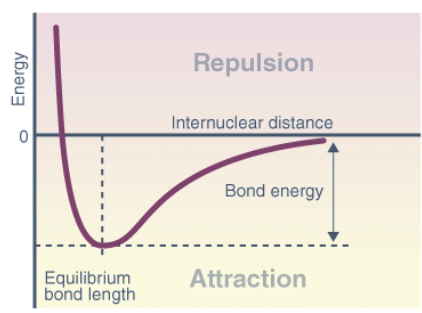
- The potential energy curve (PEC) represents the energy of a molecule as a function of the positions of its nuclei, denoted by r.
- In a system with two atoms, the energy is proportional to their separation. At longer distances, no contact exists, resulting in zero energy.
- As the distance decreases, attractive forces dominate until the atoms reach the minimum point of the curve, where attractive and repulsive forces balance.
- This minimum point determines the bond length or equilibrium bond length, which reflects the average distance at which the atoms oscillate due to thermal motion. Shorter bonds typically indicate stronger bonding.
Analysis of Potential Energy Curves
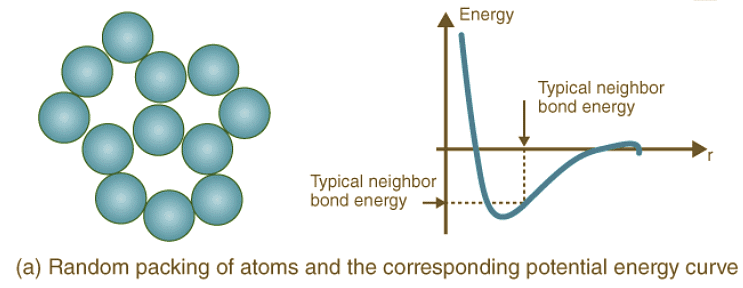
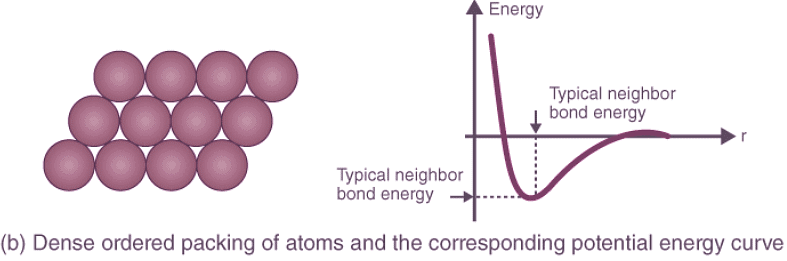
Crystal Structure Packing and Bonding Energies: Different atoms arrange themselves in various crystalline formations based on their nature, leading to diverse potential energy curves. Random and dense ordered packing of atoms exhibit contrasting potential energy curves, highlighting the role of crystal structure in determining bonding energies.
- Mechanical Properties: Mechanical characteristics define how materials deform or break under stress, time, and temperature. Understanding potential energy curves helps elucidate the behavior of materials during deformation, elongation, compression, or fracture.
- Thermal Properties: Heat capacity, thermal expansion, and thermal conductivity describe a material's response to heat. Potential energy curves provide insights into how thermal energy is absorbed and transmitted through atom vibrations in solids.
- Electrical Properties: Electrical conductivity and resistivity characterize a material's ability to conduct electricity. The energy levels and electron availability in potential energy curves influence the electrical properties of solid materials.
Conclusions
The potential energy surface and curve are valuable conceptual tools for analyzing molecular geometry and chemical reaction kinetics. The characteristics of bonding energy and the shape of potential energy curves vary from one material to another. A deep and narrow trough in the curve indicates significant bond energy, high melting temperature, large elastic modulus, and a small coefficient of thermal expansion. The diameter and asymmetry of the potential energy curve reveal distinct material properties. Different materials exhibit varying potential energy curves based on their bonding types, such as metallic bond for metals and covalent and secondary bonding for polymers.
|
48 videos|69 docs|30 tests
|















