Introduction
Electric charge is an inherent property of matter, responsible for the production and experience of electrical and magnetic effects. The conservation of charge, a fundamental principle, asserts that the total electric charge within an isolated system remains constant. In simpler terms, the net quantity of electric charge, obtained by subtracting the amount of negative charge from the amount of positive charge, always remains conserved in the universe. Similar to the conservation of energy and momentum, the conservation of charge stems from the fact that protons and electrons cannot spontaneously appear or vanish, ensuring that the total charge remains unchanged. Consequently, objects possess an equal number of electrons and protons, maintaining their overall charge balance. Moreover, atoms, the building blocks of matter, exhibit electrical neutrality, consisting of an equivalent number of electrons and protons in their nuclei. Notably, bodies can possess a charge equivalent to integral multiples of the elementary charge, which is the charge carried by an electron or proton [ie. –1.6 x 10-19 C or + 1.6 x 10-19 C]
Explanation of Conservation of Charge
The law of conservation of charge dictates that the net charge of an isolated system remains constant. Let us delve deeper into its underlying concepts. An ideal system can exist in two states: either all objects within the system possess a net neutral charge, ensuring an equal number of protons and electrons, or the net charge is uniformly distributed across the objects. In the latter scenario, rather than concentrating negative charge in a few bodies, charge transfer occurs, causing the electron to move from regions of higher polarity to regions of lower polarity. This redistribution of charge enables a uniform charge distribution throughout the system. It is crucial to note that only electrons participate in charge transfers, while protons remain fixed. By understanding these principles, we can appreciate how the conservation of charge governs the behavior of electric systems.
Examples of Conservation of Charge
The conservation of charge dictates that it is impossible to produce a net charge. Let us examine some examples illustrating this principle:
- Charges due to induction: When a charged object is brought close to an uncharged object, the uncharged object acquires an opposite charge through induction. However, the net charge of the system remains zero.
- Radioactive decay: During the decay process, a proton transforms into a positron and a neutron. Despite this transformation, the overall charge of the system does not change, exemplifying the conservation of charge.
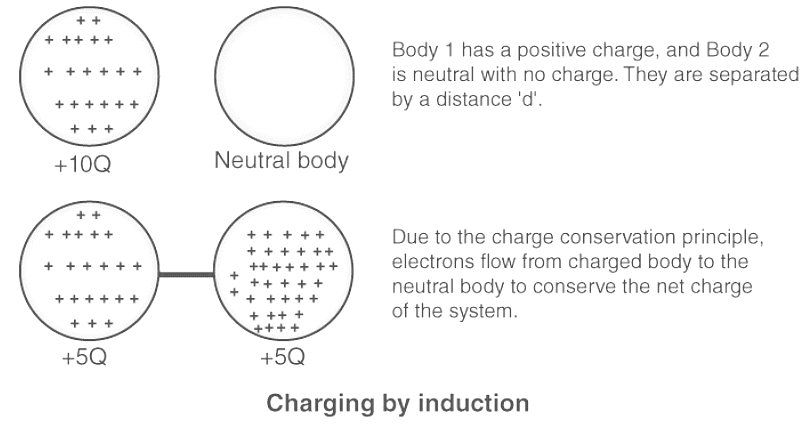
The above examples demonstrate that if a system is not influenced by external charges, the net internal charge distribution persists in a manner that ensures the overall charge of the system remains constant. Consequently, we observe that charge cannot be created or destroyed, showcasing the total conservation of charge.


















