Introduction
In the mesmerizing realm of atomic bonding, the concept of orbital overlap takes center stage. When atoms collide, they engage in a remarkable dance at the atomic level, resulting in the formation of new hybridized orbitals. This intricate process occurs when two atoms approach each other closely enough to penetrate each other's orbitals. As a consequence, a hybridized orbital is born, housing the bonding pair of electrons. These hybridized orbitals possess lower energy levels compared to their atomic counterparts, rendering them stable and residing in a state of minimum energy. The remarkable phenomenon responsible for this partial penetration is known as orbital overlap.
The Significance of Orbital Overlap: A Key to Stability and Covalent Bonds
The extent of orbital overlap hinges upon various factors such as the participating atoms, their sizes, and the valence electrons involved. A fundamental principle arises from this concept: the strength of the bond formed between two atoms is directly proportional to the magnitude of their overlap. Consequently, the orbital overlap concept asserts that atoms combine by overlapping their orbitals, generating a lower energy state where valence electrons, possessing opposite spins, unite to form covalent bonds. This pivotal concept, championed by the eminent Linus Pauling, sheds light on molecular bond angles observed through experimentation and serves as the foundation for orbital hybridization.
Directional Properties of Bond: Unraveling the Molecular Bond Angles
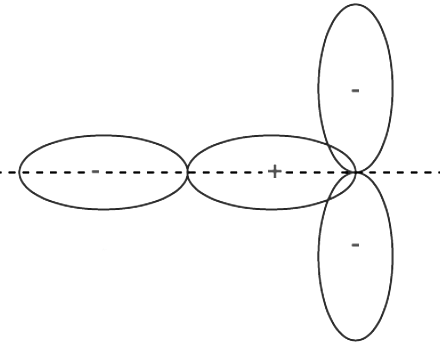
To comprehend the nuances of orbital overlap fully, one must delve into the directional properties of bonds. Examining the example of a hydrogen molecule, we witness the formation of a bond through the overlap of 1s orbitals in a head-on collision. When two atoms come into contact to establish a bond, their overlapping can assume positive, negative, or even zero values based on the phase and sign of the interacting orbitals.
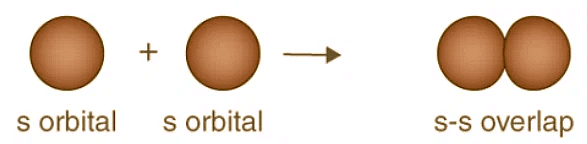
Positive Overlapping of Atomic Orbitals: The Harmony of Phases
Positive overlapping occurs when the phase of two interacting orbitals aligns. This alignment signifies that the overlap is positive, resulting in the formation of a bond. It is crucial to note that the phase of the interacting orbitals (+ or -) originates from the sign of the orbital wave function and bears no direct relationship to charge. Positive overlapping engenders stability and enables the atoms to create robust connections.
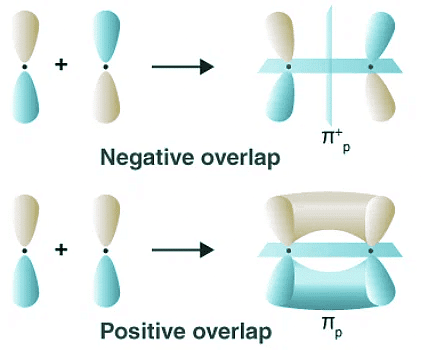
Negative Overlapping of Atomic Orbitals: A Clash of Opposites
In contrast, negative overlapping arises when two interacting atomic orbitals possess opposite phases. In this scenario, the overlap becomes negative, leading to the prevention of bond formation. The clash of opposing phases impedes the establishment of a stable connection between the atoms involved.
Zero Overlapping of Atomic Orbitals: When Paths Don't Cross
Zero overlapping manifests when the orientation of two interacting atomic orbitals fails to facilitate any overlap. In such instances, the orbitals do not intertwine efficiently or at all, resulting in a lack of bond formation. Zero overlap implies that the paths of the orbitals do not intersect, limiting their potential for cohesive bonding.