Introduction
An electrophilic substitution reaction is a chemical process in which a compound's functional group is replaced by an electrophile. Typically, the displaced functional group is a hydrogen atom.

Electrophilic substitution reactions are typically carried out in three phases, which are as follows.
- The emergence of an electrophile
- A carbocation's formation (which is an intermediate)
- The extraction of a proton from an intermediate
Electrophilic Substitution Reaction Mechanism
The electrophilic substitution reaction mechanism is composed of three steps, which will be discussed more below.
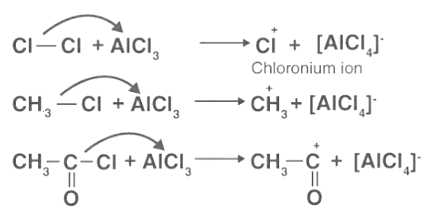
- Electrophile Generation: Anhydrous chloride is useful in the formation of electrophiles via the chlorination, alkylation, and acylation of an aromatic ring. The electrophiles formed when anhydrous aluminum chloride is combined with the attacking reagent are Cl+, R+, and RC+O, in that order.
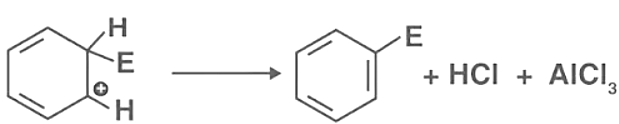
- Carbocation Formation: The electrophile then attacks the aromatic ring, forming an arenium ion or sigma complex. One of the carbons in the sigma complex will be sp3 hybridized. Part of step 2 of the electrophilic substitution reaction: The arenium ion or the sigma complex finds stability in the resonance structure. However, the aromatic feature of the sigma complex is lost because electron delocalization ends at the sp3 hybridized carbon.
- Proton Removal: When AlCl4 attacks the sigma complex or arenium ion, it releases a proton from the sp3 hybridized carbon, and this step is required to restore the aromatic property. In the third step, the electrophile replaces the hydrogen in the benzene ring.
Reaction of Amines
To begin, let us define amines. Amines are organic derivatives of ammonia (NH3) in which the hydrogen atom is substituted by alkyl, cycloalkyl, or aromatic groups to form a bond with the Nitrogen atom. Aniline is the most basic aromatic amine, consisting of amine-type nitrogen coupled to an aromatic ring.
Here are some examples of amine reactions:
- Amine, being a base, interacts with acid to generate salt.
- Alkylation occurs when amine combines as a nucleophile with alkyl halide via the substitution reaction of SN2.
- When primary aliphatic amines are oxidized by KMNO4, ethanol is produced.
Electrophilic Substitution Reaction of Anilines
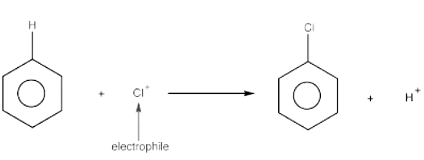
An electrophile is a species-seeking electron. Thus, an electrophilic substitution reaction occurs when one electrophile substitutes for another electrophile in an organic molecule. Halogenation, nitration, and Sulphonation are common electrophilic processes for anilines. We'll go over them one by one, but first, let's look at how anilines react to an electrophile attack:
- The functional group (-NH2) associated with aniline is an electron-donating group, which makes the electrophilic substitution process particularly active.
- The benzene ring has an excess of electrons or negative charge in the ortho- and para- positions compared to the meta-position due to its varied resonant topologies. As a result, anilines are o- and p-directive in the electrophilic substitution process.
Types of Electrophilic Substitution Reactions of Anilines
Aniline is an organic molecule with the chemical formula C6H5NH2 that consists of a phenyl group connected to an amino group. Because it is an electron-donating type group, the functional group (NH2) in aniline is particularly active in electrophilic substitution reactions. Furthermore, aniline has an excess of negative charge or electron in the ortho and para positions of a benzene ring than in the meta position; thus, the o and p locations are directed towards the electrophilic substitution process of an aniline. Aniline can execute the following electrophilic substitution reactions:
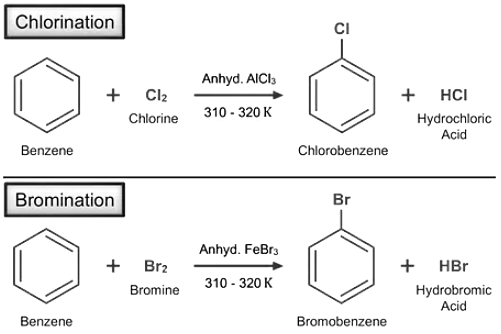
- Halogenation Reaction: The process of replacing a hydrogen atom with a halogen atom such as fluorine, chlorine, bromine, or iodine in the presence of a Lewis acid such as anhydrous aluminum chloride, ferric chloride, or ferric bromide is known as benzene halogenation.
Eg- Chlorination, and bromination
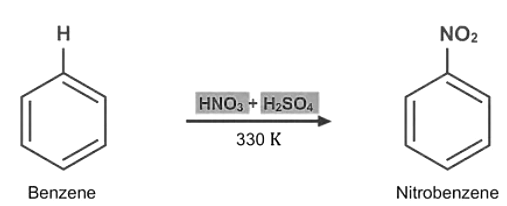
- Nitration: Nitration refers to reactions in which the hydrogen atom in the benzene ring is replaced by the nitro group. Nitration is done by heating benzene to around 330 K and adding a nitrating mixture of concentrated HNO3 and concentrated H2SO4 to it.
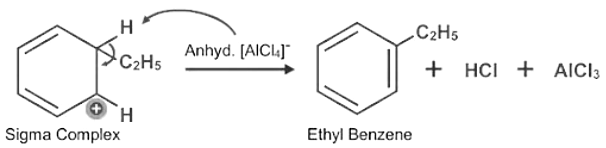
- Friedel-Crafts alkylation Reaction: Friedal-crafts alkylation reactions occur when the hydrogen atom in the benzene ring is replaced by an alkyl group in the presence of anhydrous aluminum chloride. Eg-In the presence of anhydrous aluminum chloride, benzene combines with ethyl chloride to create ethylbenzene.
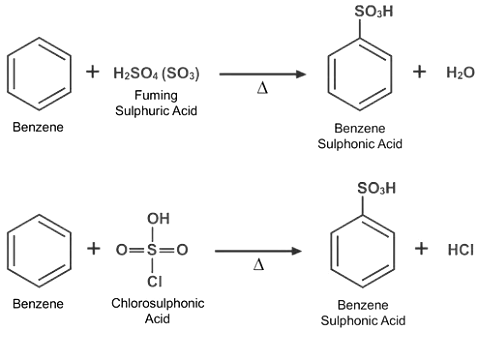
- Sulphonation Reaction: Sulphonation occurs when the hydrogen atom in a benzene ring is replaced by the sulphonic acid (-SO3H) group. Heat Benzene with fuming sulphuric acid or oleum to initiate the sulphonation reaction. Treatment of benzene with chloro sulphonic acid can also be used to sulphonate it.
Benzene interacts with hydrogen at 473 to 573 K under pressure in the presence of a catalyst such as nickel or platinum to create cyclohexane.
Points to Remember:
- Unique Electrophile: H+ is an exceptional electrophile since it lacks electrons and can only accept them.
- Water as Both Electrophile and Nucleophile: Water (H2O) exhibits electrophilic and nucleophilic properties due to the oxygen atom's high electronegativity, possessing two lone pairs and a partial negative charge (d-), while the hydrogen atoms carry a partial positive charge (d+).
- Electrophilic Substitution Reactions: Electrophilic substitution reactions involve the replacement of a functional group in a compound, often a hydrogen atom, with an electrophile.
- Amines and Substitution: Amines are organic compounds derived from ammonia (NH3), where one or more hydrogen atoms are replaced by alkyl, cycloalkyl, or aromatic groups, forming bonds with the nitrogen atom.
- Aniline Structure and Electrophilic Substitution: Aniline (C6H5NH2) is an organic molecule consisting of a phenyl group connected to an amino group. The ortho and para positions on the benzene ring of aniline contain an excess of negative charge or electron density compared to the meta position. Consequently, these ortho and para positions are more susceptible to undergoing electrophilic substitution reactions.














