Introduction
In the realm of organic chemistry, nucleophilic addition reactions hold tremendous significance. These chemical reactions involve the formation of a sigma bond between a nucleophile and an electron-deficient species. They play a crucial role in the conversion of carbonyl groups into a wide range of functional groups, making them a fundamental concept to grasp. In this article, we will delve into the general mechanism behind nucleophilic addition reactions and explore several captivating examples that showcase their remarkable versatility.
Understanding the General Mechanism
Nucleophilic addition reactions of carbonyl compounds can be broken down into three key steps:
- Formation of a Sigma Bond: The electrophilic carbonyl carbon initiates a sigma bond with the nucleophile, establishing a vital connection.
- Breaking of the Carbon-Oxygen Pi Bond: The carbon-oxygen pi bond is cleaved, resulting in the formation of an alkoxide intermediate. During this process, the bond pair of electrons is transferred to the oxygen atom.
- Protonation and Derivative Formation: The alkoxide intermediate undergoes protonation, leading to the generation of an alcohol derivative.
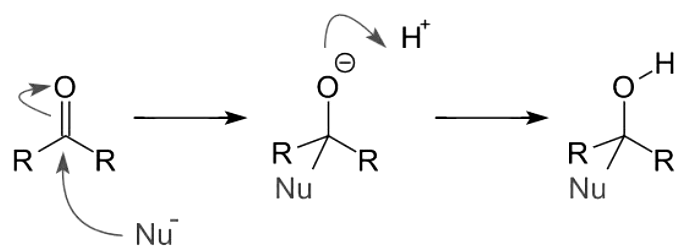
It's important to note that when strong nucleophiles attack the carbon-oxygen double bond directly, the alkoxide is formed without the need for additional activation. However, when weak nucleophiles are involved, an acid catalyst becomes essential to activate the carbonyl group for the nucleophilic addition reaction to proceed.
What is the reason for nucleophilic addition in carbonyl compounds?
Carbonyl compounds exhibit a polar carbon-oxygen bond due to the higher electronegativity of the oxygen atom, resulting in increased electron density near the oxygen. Consequently, the oxygen atom acquires a partial negative charge, while the carbon atom gains a partial positive charge.
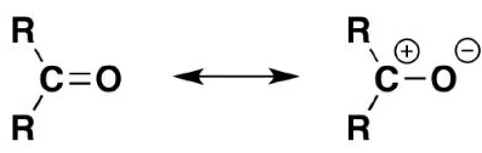
The partial positive charge on the carbonyl carbon makes it an electrophile. The introduction of an acidic group helps stabilize the partial negative charge on the oxygen atom. The acidic group donates a proton, which forms a bond with the carbonyl oxygen atom, neutralizing the negative charge. Compared to ketones, aldehydes exhibit relatively higher reactivity towards nucleophilic addition reactions. This is because the adjacent R groups stabilize the secondary carbocations formed by ketones. In contrast, the primary carbocations formed by aldehydes are less stable than the secondary carbocations of ketones, making them more prone to nucleophilic attacks.
Reactions involving Hydrogen Cyanide
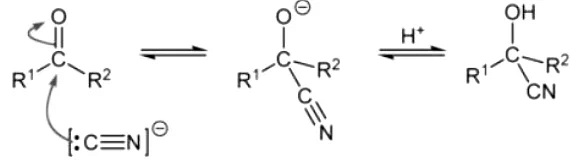
- When hydrogen cyanide (HCN) reacts with carbonyl compounds, typically aldehydes and ketones, a nucleophilic addition reaction takes place resulting in the formation of cyanohydrins. To enhance the reaction rate, base catalysts are commonly employed. The cyanide anion (CN–) acts as a potent nucleophile, attacking the carbonyl carbon and forming a new sigma bond, as depicted below.
- The electrophilic nature of the carbonyl carbon arises from the polar nature of the C=O bond. The cyanide anion acts as a nucleophile, initiating an attack on the carbonyl carbon and generating an intermediate. This intermediate is subsequently protonated to yield the cyanohydrin product.
Nucleophilic Additions with Monohydric Alcohols
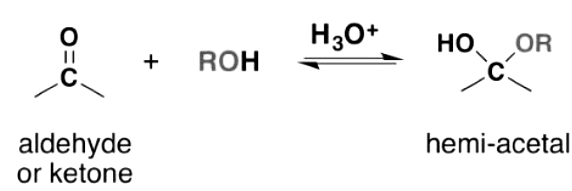
- Aldehydes and ketones undergo nucleophilic addition reactions with monohydric alcohols, resulting in the formation of hemiacetals. Upon further reaction with another alcohol molecule, an acetal is formed. Since alcohols are weak nucleophiles, the reaction necessitates an acid catalyst to activate the carbonyl group for nucleophilic attack.
- Due to the hydrolysis capability of hemiacetals, leading to the regeneration of the reactants (alcohol and carbonyl compound), any water formed during the reaction must be eliminated. In this reaction, the carbonyl oxygen is protonated prior to the nucleophilic attack by the alcohol. The nucleophilic alcohol is subsequently deprotonated, resulting in the formation of the hemiacetal. This process can be repeated to obtain the acetal product.
Other Examples
Nucleophilic Addition with Grignard Reagents
Grignard reagents, which have a general formula of R-Mg-X, exhibit a partial negative charge on the carbon atom due to their polar nature. When these reagents undergo nucleophilic addition reactions, they lead to the formation of various types of alcohols:
- Formaldehyde reacts with Grignard reagents to produce primary alcohols.
- Secondary alcohols are obtained when other aldehydes react with Grignard reagents.
- Nucleophilic addition reactions between ketones and Grignard reagents result in the formation of tertiary alcohols.
The general mechanism for these reactions involves the nucleophilic attack of the carbon atom (belonging to R-Mg-X) on the carbonyl carbon. Upon subsequent acid workup, the resulting alkoxide is converted into the corresponding alcohol.
Primary Amines Reacting with Aldehydes and Ketones
The reaction between primary amines and aldehydes/ketones produces imine derivatives along with water. The process can be described as follows:
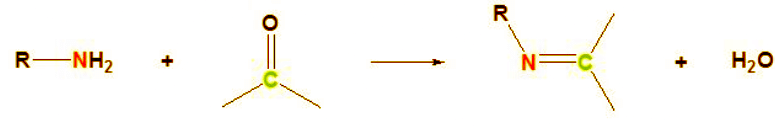
Initially, the nucleophilic nitrogen from the amine attacks the carbonyl carbon, breaking the carbon-oxygen double bond and forming a new carbon-nitrogen sigma bond. Subsequently, a proton is transferred from the amine to the oxygen atom. In the next step of this nucleophilic addition reaction, the OH group undergoes further protonation and water is eliminated. Consequently, the carbon atom forms a double bond with the nitrogen from the amine. The nitrogen is then deprotonated, resulting in the desired imine product.














