Class 7 Science Chapter 5 Question Answers - Physical and Chemical Changes
Q1. Write the differences between physical and chemical changes.
Ans:
- Physical changes and chemical changes are different in many ways. A physical change does not produce a new substance.
- Examples of this could be changing the shape of a substance, like cutting a piece of paper, or changing the state of a substance, like melting an ice cube.
- A chemical change, on the other hand, does result in a new substance being produced. This could be something like burning wood, where the wood turns into ash, or an egg turning into a chick.
Q2. In addition to the formation of new products, what changes do the chemical changes accompany?
Ans:
Chemical changes result in new products, but they also often result in other changes as well. These could include:
- Heat, light or any other radiation (e.g. ultraviolet) may be given off or absorbed.
- Sound may be produced.
- A change in smell may take place or a new smell may be given off.
- A colour change may take place.
- A gas may be formed.
Q3. Which type of change takes place in the following and state whether the energy is evolved or absorbed during the change?
Burning of a candle, lightning of a bulb, preparation of food by green plants, volcanic eruption, evaporation of petrol, burning of LPG.
Ans:
- When a candle burns, it's both a physical and chemical change. The wax melts, which is a physical change, but the wick also burns and produces heat and light, which are signs of a chemical change.
- When a bulb lights up, it's a physical change because the filament in the bulb heats up and glows, but doesn't produce a new substance.
- When plants prepare food, it's a chemical change because they use light to turn carbon dioxide and water into glucose, a new substance.
- A volcanic eruption is a chemical change because it releases gases and molten rock, which are new substances.
- When petrol evaporates, it's a physical change because the petrol just changes from a liquid to a gas, but doesn't become a new substance.
- When LPG burns, it's a chemical change because it produces carbon dioxide and water, which are new substances.
Q4. Describe two changes that are harmful. Explain why you consider them harmful? How can you prevent them?
Ans:
Harmful changes are
- Rusting of iron: Rusting of iron is harmful because it slowly destroys iron articles and makes them useless. Since, iron is used in making large number of objects or articles such as bridges, grills, railings, gates and bodies of cars, buses, trucks and ships, etc. Rusting of iron causes a great loss over a period of time. Prevention Rusting can be prevented by oiling, greasing or painting. It can also be prevented by galvanisation.
- Decaying of fruits: Decaying of fruits causes health hazards. Due to decaying of fruits, there is a lot of monetary loss in food industry. Prevention Fruits can be preserved by keeping them at low temperature and by using some specific preservatives.
Q5. Explain the burning of magnesium ribbon.
Ans: Take a thin ribbon of magnesium. Gently clean the end of the ribbon with sand paper and bring its tip near a candle flame. It is observed that the ribbon burns with a bright white light. After combustion, white powdery ash is left, which is called magnesium oxide.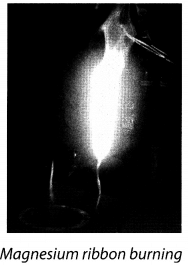
Q6. What are the characteristics of a chemical change?
Ans: The characteristics of a chemical change are:
- They release or absorb energy.
- Most of the changes are irreversible.
- One or more new substances with new properties are formed.
- The properties of products are entirely different from the reactants.
Q7. Why is the burning of a candle considered a chemical change?
Ans: Candles are made of wax and a long thread of cotton (called wick of the candle). While candle is burnt, the molten wax goes up through the thread and undergoes combustion to form carbon dioxide and water vapour. The ‘wick’ of the candle gets changed to a black mass.
Over the process, heat and light energy is given out. It is not possible to
(i) recover the burnt wax again,
(ii) recover the thread again.
Hence, the burning of a candle is a chemical change.
Q8. What happens when iron nails are dipped in copper sulphate solution?
Ans: When iron nails are dipped in copper sulphate solution, a brown layer of copper gets deposited on the surface of iron nails after some time. This happens due to the reaction between copper sulphate and iron. Also, the colour of copper sulphate changes from blue to green due to the formation of iron sulphate.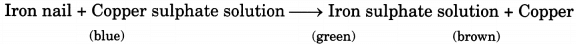
Q9. Give an example of a chemical reaction for each of the following situations:
(a) A change in colour is observed.
(b) A gas is evolved.
(c) Sound is produced.
(d) Heat is produced.
(e) Change in taste is observed.
(f) Light is produced.
Ans:
(a) Reaction between copper sulphate solution and iron metal. Blue colour of copper sulphate solution changes to green colour ferrous sulphate solution.
(b) Reaction between baking soda and vinegar evolves carbon dioxide gas.
(c) Burning of crackers produces sound.
(d) Reaction between hydrochloric acid and sodium hydroxide produces heat.
(e) Setting of curd from milk. Taste of milk changes to sour in curd.
(f) Burning of fuel produces light.
Q10. Most of the physical changes are reversible but some are irreversible. Explain the statement with examples.
Ans: In physical change, a substance undergoes a change in its physical properties only. Physical change occurs when there is a force applied, change in temperature, etc., on a substance. Most of the physical changes are reversible like rolling a dough into a chapati and then again bringing back into a dough.
Making a toy aeroplane by folding the paper and then again unfolding the toy aeroplane to recover the page. But when a physical change cannot be reversed or we cannot bring the substance back to its original shape, size, state, etc., is known as irreversible change. For example, baking chapati or making a toy aeroplane by cutting the paper instead of folding it.
|
111 videos|286 docs|28 tests
|





















