Class 7 Science Chapter 3 HOTS Questions - Heat
Q1. Two objects at the same temperature, one smaller than the other, are placed together. In which direction will the heat be transferred? Give reason.
Ans: There will be no heat transfer between the two objects because:
- Heat flows only from a higher-temperature object to a lower-temperature object.
- Since both objects are at the same temperature, there is no temperature difference.
- As a result, no heat flow occurs, regardless of their size.
Q2. In a mercury thermometer, the level of mercury rises when its bulb comes in contact with a hot object. What is the reason for this rise in the level of mercury?
Ans: When the bulb of a mercury thermometer touches a hot object, heat transfers from the object to the mercury. This causes the mercury to:
- Expand due to the increase in temperature.
- Rise in the capillary tube, indicating a higher temperature.
Q3. The radiators in cars are painted black. Explain why.
Ans: The radiators in cars are painted black. Car radiators are painted black for the following reasons:
- Black surfaces are more effective at radiating heat.
- Painting them black helps to improve heat dissipation.
- This ensures the engine stays cool and operates efficiently.
Q4. Why are the pipes of the solar heater and the containers of the solar cooker painted black?
Ans: The pipes of the solar heater and the containers of the solar cooker are painted black because:
- Black colour absorbs more heat than other colours.
- This helps to keep the pipes and containers warm.
Q5. At a campsite, there are tents of two shades. One is made with black fabric and the other with white fabric. Which one will you prefer for resting on a hot summer afternoon? Give a reason for your choice. Would you like to prefer the same tent during winter?
Ans: For resting on a hot summer afternoon, a white fabric tent is preferable because it reflects sunlight, keeping the interior cool. In winter, however, a black fabric tent is more suitable as it absorbs sunlight, helping to maintain warmth inside.
Q6. Explain the reason for the following statement, “When heat is applied at the bottom of the water vessel, then it gets heated more quickly than when it is heated at the top.”
Ans: The reason for the statement is related to the movement of water particles when heated:
- When heat is applied at the bottom of the vessel, the water particles near the heat source become lighter and rise.
- As the hot water rises, the colder and heavier water from the sides moves down to replace it.
- This creates convection currents, allowing the water to heat up more efficiently.
- Conversely, when heat is applied at the top, only the upper layer of water heats up, while the hot water remains at the surface.
Q7. The freezer is located at the top of the refrigerator. Explain why.
Ans: The freezer is positioned at the top of the refrigerator for the following reasons:
- Cold air is denser than warm air, causing it to sink.
- This allows the cool air from the freezer to circulate downwards.
- As a result, the refrigerator section below is effectively cooled.
- This design enhances the overall cooling efficiency of the appliance.
Q8. To keep her soup warm, Paheli wrapped the container in which it was kept with woollen clothes. Can she apply the same method to keep a glass of cold drink cool? Give a reason for your Answer.
Ans: Yes, she can use the same method to keep a glass of cold drink cool because:
- Wool is a thermal insulator.
- It prevents heat from passing through.
- This helps maintain the cold temperature of the drink.
Q9. A laboratory thermometer A is kept 7 cm away on the side of the flame while a similar thermometer B is kept 7 cm above the flame of a candle as shown in the figure. Which of the thermometers A or B will show a greater rise in temperature? Give a reason for your answer.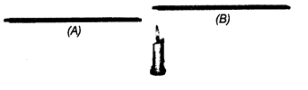
Ans: Thermometer B will show a greater rise in temperature because:
- The heated air above the candle rises quickly.
- This increases the temperature of the bulb of thermometer B more than that of thermometer A.
Q10: A circular metal loop is heated at point O as shown in the figure.
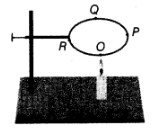
(a) In which direction, would heat flow in the loop?
(b) In which order, are the pins at points P, Q and R fixed with the help of wax fall if points O, P, Q and R are equidistant from each other?
Ans:
(a) Heat will flow in both directions from O to P and from O to R.
(b) First of all, the pin at P and R will fall simultaneously. After that, the pin at Q will fall.
Q11. You may have noticed that a few sharp jerks are given to a clinical thermometer before using it. Why is it done so?
Ans: Jerks are given to a clinical thermometer before use to:
- Lower the mercury level below normal temperature.
- Ensure an accurate measurement of body temperature.
Q12. For setting curd, a small amount of curd is added to warm milk. The microbes present in the curd help in setting if the temperature of the mixture remains approximately between 35°C to 40°C. At places where room temperature remains much below the range, setting of curd becomes difficult. Suggest a way to set curd in such a situation.
Ans: To set curd in cooler conditions, follow these steps:
- Use a thermally insulated cover for the container.
- Wrap the container with wool or jute sacks to maintain warmth.
- Ensure the mixture stays at a temperature between 35°C and 40°C.
This helps create a suitable environment for the curd to set properly.
Q13: A beggar wrapped himself with a few layers of newspaper on a cold winter night. This helped him to keep himself warm because
(a) friction between the layers of newspaper produces heat
(b) air trapped between the layers of newspaper is a bad conductor of heat
(c) newspaper is a conductor of heat
(d) newspaper is at a higher temperature than the temperature of the surroundings
Ans: (b) air trapped between the layers of newspaper is a bad conductor of heat
The beggar used layers of newspaper to keep warm on a cold winter night due to the following reasons: The air trapped between the layers of newspaper acts as an insulator, preventing heat loss. Newspaper itself is not a good conductor of heat, which helps maintain warmth.
Q14. Shopkeepers selling ice blocks usually cover them with jute sacks or sawdust. Explain why.
Ans: Jute sacks or sawdust are commonly used to cover ice blocks for the following reasons:
- They act as insulating materials, helping to maintain the temperature of the ice.
- This insulation reduces the rate at which the ice melts.
- By covering ice blocks, shopkeepers can ensure that the ice lasts longer for customers.
Q15. Why are milk vans carrying milk from the factory to the depots painted silver or white?
Ans: Milk vans are painted silver or white for several reasons:
- Light Reflection: White surfaces reflect maximum light, helping to keep the interior cool.
- Heat Absorption: These colours absorb minimal heat, which is crucial for maintaining the freshness of the milk.
- Temperature Control: By reducing heat absorption, the milk remains at a stable temperature during transport.
Q16. Why does the level of mercury rise when its bulb comes in contact with a hot object?
Ans: Mercury expands when heated, causing its level to rise in the capillary tube of the thermometer.
Q17. While constructing a house in coastal area, in which direction should the windows preferably face and why?
Ans: The windows should ideally face towards the sea for the following reasons:
- The sea breeze helps to keep the house cool during the day.
- This positioning allows for better ventilation and comfort.
At night, the situation reverses, as the land cools faster than the sea, leading to a land breeze that can also be beneficial.
Q18. Ventilators are situated close to the ceilings and not near the floor. Why?
Ans: Ventilators are placed near the ceilings rather than the floor for several reasons:
- Warm air is lighter than cool air, causing it to rise.
- As warm air rises, it creates a vacuum that pulls in cooler air from below.
- Ventilators at the top help to circulate fresh air effectively.
- This design enhances overall air quality in the room.
|
112 videos|279 docs|28 tests
|
FAQs on Class 7 Science Chapter 3 HOTS Questions - Heat
| 1. What is heat and how does it transfer? |  |
| 2. What is the difference between temperature and heat? |  |
| 3. How is heat measured and what units are used? |  |
| 4. What is specific heat and why is it important? |  |
| 5. How does heat affect states of matter? |  |