Class 9 Science Chapter 4 Question Answers - Structure of the Atom
Q1: On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Ans: As per Thomson’s model of an atom, the number of electrons and the number of protons are equal in an atom. Electrons are positively charged and protons are negatively charged, hence the + and – charges are neutralized by each other that makes atoms neutral as a whole.
Q2: On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Ans: The subatomic particle present in the nucleus of an atom, according to Rutherford's model, is the proton.
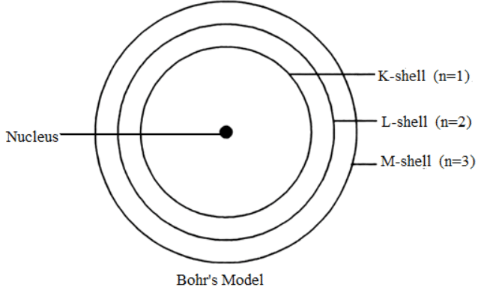
Q3: Draw a sketch of Bohr’s model of an atom with three shells.
Ans: Bohr’s model of an atom with three shells is as follows:
Q4: What do you think would be the observation if the α− particle scattering experiment is carried out using a foil of a metal other than gold?
Ans: If the α− particle scattering experiment is carried out using a foil of a metal other than gold we will get a different observation. Overall, the results would depend on the properties of the metal used.
Q5: Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Ans: The atomic mass of an atom is the sum of masses of protons and neutrons present in its nucleus.
Given that the mass of the helium atom is 4 u and two protons present in its nucleus.
So the number of neutrons will be
Number of neutrons = atomic mass − number of protons
⇒ Number of neutrons = 4−2
∴ Number of neutrons = 2
Therefore, the helium atom has 2 neutrons.
Q6: Write the distribution of electrons in carbon and sodium atoms.
Ans: Distribution of electrons in carbon and sodium atoms is as follows:
Carbon:
- Atomic number: 6
- Electron distribution: 2, 4
Sodium:
- Atomic number: 11
- Electron distribution: 2, 8, 1
Q7: If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Ans: K shell contains total 2 electrons and L shell contains maximum 8 electrons. If K and L shells of an atom are full, then the total number of electrons in the atom will be 10.
Q8: If number of electrons in an atom is 8 and number of protons is also 8, then
(a) What is the atomic number of the atom?
Ans: The atomic number of an atom is equal to the number of protons or electrons present in its nucleus. So the atomic number of an atom with 8 electrons and 8 protons is 8.
(b) What is the charge on the atom?
Ans: A single electron contains one negative charge and one single proton contains one positive charge. There are equal numbers of electrons and protons in an atom so they neutralize each other. The atom will be neutral.
Q9: Write the electronic configuration of following ions:
(a) Cl−
Ans: Electronic configuration of Cl− ion is 2,8,8.
(b) Mg
Ans: Electronic configuration of Mg ion is 2,8,2.
(c) Al3+
Ans: Electronic configuration of Al3+ ion is 2,8.
(d) O
Ans: Electronic configuration of O is 2,6.
Q10: What are the limitations of J.J. Thomson’s model of the atom?
Ans: The J.J. Thomson’s atomic model failed to explain the organization of electrons in an atom.
Q11: Na+ has completely filled K and L shells. Explain.
Ans: Sodium (Na) has atomic number 11, so the electronic configuration of Na is 2,8,1.
It has a single electron in the outermost shell, when it gives away that electron it becomes Na+ and has electronic configuration 2,8. Also the K shell contains a total 2 electrons and the L shell contains a maximum of 8 electrons. So Na+ has completely filled K and L shells.
Q12: If z = 3, what would be the valency of the element? Also, name the element.
Ans: z = 3 represents that element has 3 electrons in its shells. The electronic configuration is 2,1. It means the outermost shell electron has 1 electron, so its valency is 1. The element is Lithium.
Q13: For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
Ans: False
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
Ans: True
(c) The mass of an electron is about 12000 times that of proton.
Ans: True
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Ans: False
Q14: Explain the formation of Al3+ ion and why is it formed?
Ans: Aluminum has an atomic number of 13. The electronic configuration of Al is 2,8,3. It has 3 electrons in the outermost shell and to become stable it needs to complete its octet. In the outermost shell, the maximum number of electrons must be 8. So it is easy to lose 3 electrons and complete the octet. By giving away the 3 outermost electrons it becomes Al3+ ion.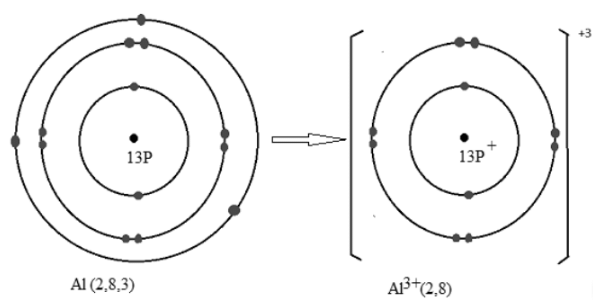
Q15: Why metals are electropositive and non-metals are electronegative in nature?
Ans: Metals are electropositive in nature because all metals give away electrons from their outermost shell in order to complete the octet and become stable. So metals become positively charged.
Non-metals are electronegative in nature because all non-metals gain electrons in order to complete the octet and become stable. So non-metals become negatively charged.
Q16: Write the postulates of Bohr theory?
Ans: The postulates of Bohr's theory are as follows:
- Electrons revolve around the nucleus in specific paths called orbits or shells.
- Each orbit has a fixed amount of energy.
- The energy increases from the inner shells to the outer shells, with the innermost shell having the lowest energy.
- If energy is supplied, an electron can move from a lower orbit to a higher orbit.
Q17: Compare the three major particles in atoms with respect to their mass and charge?
Ans: Comparison of three major particles proton, neutron and electron with respect to their mass and charge is as follows: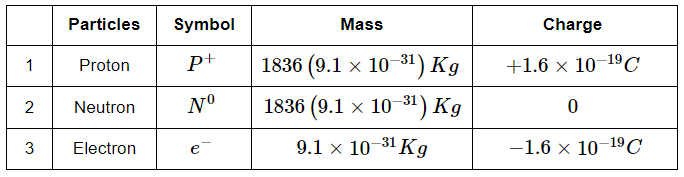
Inside an atom electron revolves around the nucleus in a circular path. Protons and neutrons are present inside the nucleus.
Q18: In a gold – foil experiment:
(a) Why did many α− particles pass through the gold foil undeflected?
Ans: Most of the space within the atom was empty so many α− particles passed through the gold foil undeflected.
(b) Why did few α− particles deflect through small angles.
Ans: In a gold foil at center there is a positive charge so few α− particles deflect through small angles.
(c) Why did few α− particles, after striking the gold foil, retrace their path.
Ans: Very few α-particles retraced their path after striking the foil, indicating that the positively charged nucleus is very small.
Q19: Define valency by taking examples of silicon and oxygen.
Ans: The valency of electrons is determined by electrons present in the outermost shell of an atom. Electrons present in the outermost shell of an atom are known as the valence electrons. Electrons gain or lose electrons to complete its octet.
Atomic number of silicon is 14 and electronic configuration is 2,8,4. This means it can either gain or lose 4 electrons. So the valency of silicon is 4.
Atomic number of oxygen is 8 and the electronic configuration is 2,6. To complete its octet oxygen gains 2 electrons hence the valency of oxygen is 2.
Q20: The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 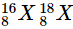 and in the sample?
and in the sample?
Ans: Average atomic mass of sample is given as
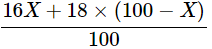
We get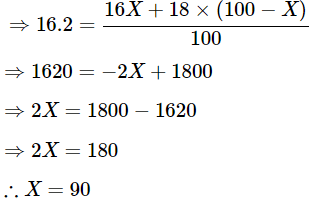
The percentage of isotopes is 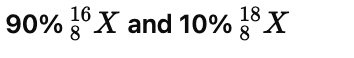
Q21: Define isotopes.
Ans: Isotopes are atoms which have identical atomic numbers but different mass numbers. Examples of isotopes are 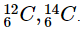
Q22: Which of the following electronic configuration are wrong and why?
(a) 2,8,2
(b) 2,8,8,2
(c) 2,8,9,1.
Ans: From the given electronic configuration, 2,8,9,1 is wrong because in the third shell the maximum number of electrons is 8. The correct electronic configuration is 2,8,8,2.
Q23: What are ions? What are its two types?
Ans: When one or more electrons are detached from a neutral atom, a positively charged particle is formed and called an ion. Ions may be cations and anions.
Q24: Show diagrammatically the formation of O2− ion?
Ans: Atomic number of oxygen is 8 and its electronic configuration is 2,6. In the outermost shell oxygen has 6 electrons. To complete its octet and become stable it needs 2 electrons. By gaining 2 electrons it becomes O2− ion.
Diagrammatic representation of formation of O2− ion is as follows: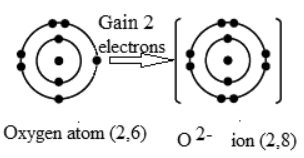
Q25: Define isobars.
Ans: Isobars are atoms that have different atomic numbers but the same mass number. Examples of isobars are 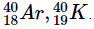
Q26: For the symbol H, D and T tabulate three subatomic particles found in each of them.
Ans: H represents the hydrogen atom, D represents the deuterium atom and T represents the tritium atom. Three subatomic particles present in each of them is represented as follows: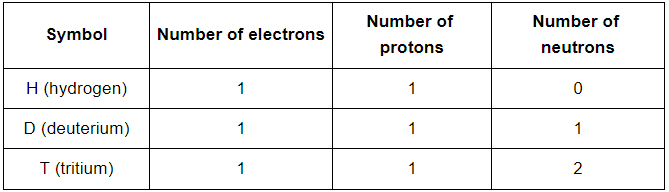
Q27: Write the electronic configuration of any one pair of isotopes and isobars.
Ans: Electronic configuration of pairs of isotopes of carbon is 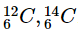 . Isotopes have the same number of electrons and protons.
. Isotopes have the same number of electrons and protons.
Electronic configuration of a pair of isobars of argon and calcium is 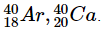
Q28: Compare the properties of electrons, protons and neutrons.
Ans: Comparison of electrons, protons and neutrons is as follows:
Q29: What are the limitations of Rutherford’s model of the atom?
Ans: Rutherford's model of the atom has several limitations:
- The model does not explain the stability of atoms. It suggests that electrons orbit the nucleus, which would cause them to emit energy.
- As electrons lose energy, their orbits would shrink, eventually leading them to collide with the nucleus. This implies that atoms should be unstable, contradicting the observed stability of matter.
Q30: If bromine atom is available in the form of, say, two isotopes 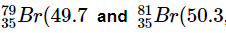 Calculate the average atomic mass of bromine atom.
Calculate the average atomic mass of bromine atom.
Ans: The average atomic mass of bromine is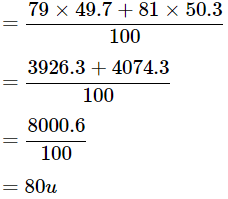
Average atomic mass of bromine atoms is 80 u.
|
84 videos|384 docs|61 tests
|
FAQs on Class 9 Science Chapter 4 Question Answers - Structure of the Atom
| 1. What are the main components of an atom? |  |
| 2. How do protons and neutrons differ from electrons? |  |
| 3. What is the significance of atomic number and mass number? |  |
| 4. What is an isotope, and how does it differ from a regular atom? |  |
| 5. How do atomic models explain the structure of the atom? |  |






















