4 Days Timetable: Chemical Reactions and Equations | Science Class 10 PDF Download
| Table of contents |

|
| Day 1: Chemical Equations |

|
| Day 2: Types of Chemical Reactions |

|
| Day 3: Effects of Oxidation Reactions in Everyday Life |

|
| Day 4: Revision |

|
The chapter on "Chemical Reactions and Equations" is a crucial part of the Class 10 Science curriculum and holds significant importance in the board exam. It is imperative to understand this chapter thoroughly as it forms the basis for more advanced topics in chemistry. This chapter encompasses three major topics: Chemical Equations, Types of Chemical Reactions, and Effects of Oxidation Reactions in Everyday Life. A strong grasp of these concepts is essential not only for your board exam but also for a deeper understanding of chemistry.
You can adjust the timetable according to your pace, but the pattern of covering the topics should remain the same. Let's dive into the study plan.
Topics to Cover
Before we begin, let's briefly outline the topics we need to cover in this chapter:
- Chemical Equations
- Types of Chemical Reactions
- Effects of Oxidation Reactions in Everyday Life
Now, let's break down the study plan into four days of focused learning and one day dedicated to revision.
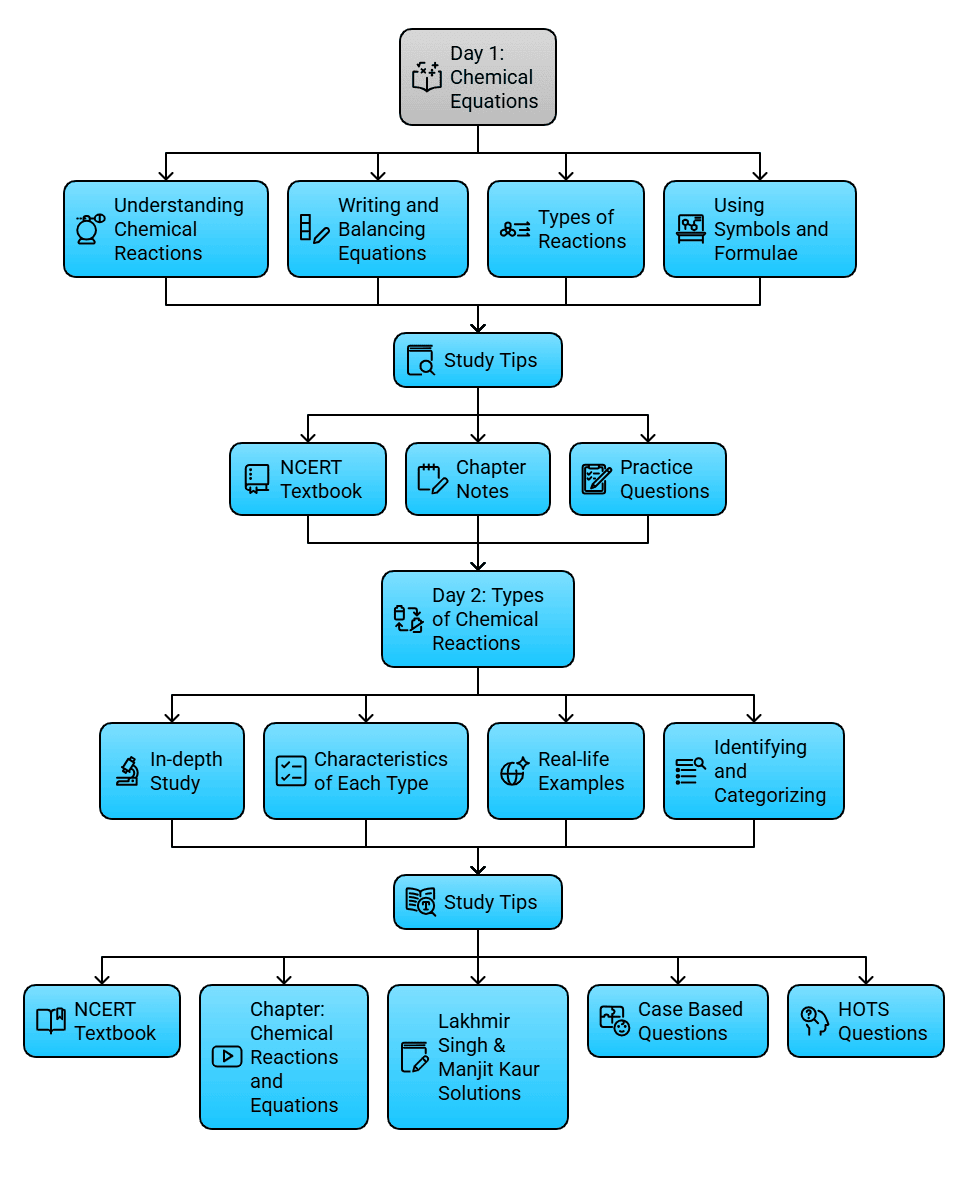
Day 1: Chemical Equations
What to Cover:
- Understanding chemical reactions and their importance.
- Writing and balancing chemical equations.
- Types of chemical reactions: Combination, Decomposition, Displacement, and Double Displacement.
- Using symbols and formulae to represent elements and compounds in equations.
Study Tips:
- Start by reading the relevant NCERT textbook chapters.
- Refer to the Chapter Notes on EduRev for concise notes and examples.
- Practice writing and balancing chemical equations.
- Solve the questions from the Unit Test and Practice Questions on EduRev to test your understanding.
Day 2: Types of Chemical Reactions
What to Cover:
- In-depth study of different types of chemical reactions.
- Understanding the characteristics of each type: Combination, Decomposition, Displacement, and Double Displacement.
- Real-life examples of these reactions.
- Identifying and categorizing reactions based on their type.
Study Tips:
- Review the NCERT textbook for a comprehensive understanding.
- Explore the Chapter: Chemical Reactions and Equations on EduRev for videos and additional resources.
- Practice categorizing reactions by solving questions from the Lakhmir Singh & Manjit Kaur Solutions on EduRev.
- Test your knowledge with the Case Based Questions and HOTS Questions on EduRev.
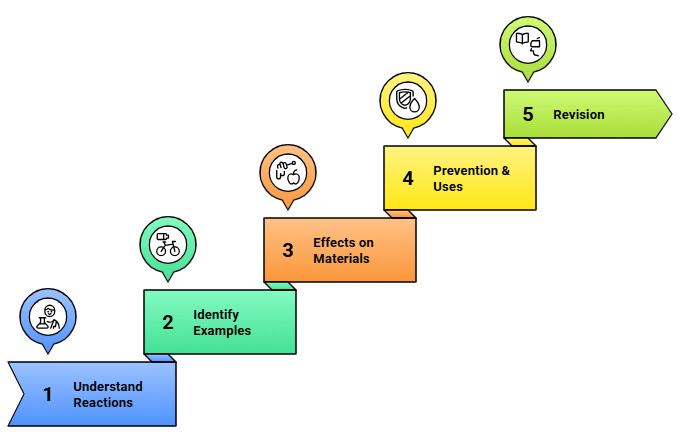
Day 3: Effects of Oxidation Reactions in Everyday Life
What to Cover:
- Understanding oxidation reactions and their significance.
- Identifying common oxidation reactions in daily life.
- Effects of oxidation on various materials, such as metals and food.
- Prevention and uses of oxidation reactions.
Study Tips:
- Carefully read the relevant NCERT chapters.
- Explore the Mindmap on EduRev for a visual summary.
- Solve the Assertion & Reason Type Questions to enhance your problem-solving skills.
- Practice with the Practice Questions on EduRev for a thorough understanding.
Day 4: Revision
On this day, focus on revising all the topics you've covered so far. Use the following resources:
- Go through the Short & Long Questions & Answers on EduRev to recapitulate key concepts.
- Test yourself with Topic Wise tests on EduRev for each of the three topics.
- Practice solving questions of different types: Very Short Answer type questions, Short Answer type Questions, and Long Answer Type Questions to assess your preparation thoroughly.
In your Class 10 Science board exam, you will encounter questions that require a deep understanding of chemical reactions and equations. The best way to prepare for these questions is by solving various types of questions and practicing consistently. Remember to make use of the resources available on EduRev, such as videos, notes, and practice questions, to strengthen your knowledge.
By following this structured study plan and regularly testing your knowledge with the provided resources, you can confidently tackle the chapter on "Chemical Reactions and Equations" and excel in your Class 10 board exam.
For comprehensive study materials and additional resources for Class 10 Science, explore Class 10 Boards on EduRev and make the most of your exam preparation.
Here are all the important links and topic-specific links for the chapter on "Chemical Reactions and Equations":
Important Links:
- Class 10 Boards on EduRev
- Past Year Questions
- Lakhmir Singh & Manjit Kaur Solutions
- NCERT Exemplar
- Flowcharts & Important terms
- Worksheet with Solutions
Topic-Specific Links:
- Chemical Equations: Writing & Balancing
- Chemical Reactions: Types, Corrosion & Rancidity
- Oxidation & Reduction
These links will help you access specific resources and materials related to each topic within the chapter, making your preparation more effective and comprehensive.
Good luck!
|
82 videos|677 docs|80 tests
|
FAQs on 4 Days Timetable: Chemical Reactions and Equations - Science Class 10
| 1. What is a chemical equation? |  |
| 2. What are the different types of chemical reactions? |  |
| 3. How do oxidation reactions affect everyday life? |  |
| 4. What are some examples of chemical equations in everyday life? |  |
| 5. How can chemical equations be balanced? |  |
















