Diagram Based Questions: Acids, Bases and Salts | Science Class 10 PDF Download
Q1: Answer the following questions based on the diagram given below:
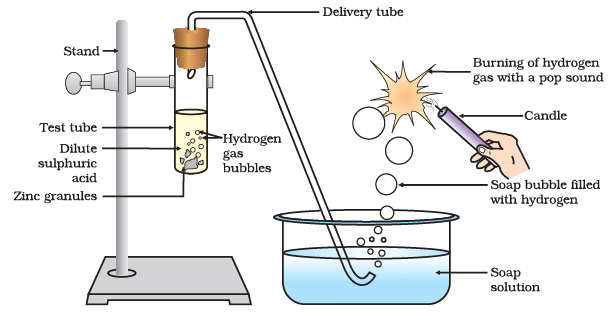
(i) What are the reactants in the experiment, and what does it produce?
Ans: In the experiment, zinc granules are the reactant, and when they react with dilute sulfuric acid, they produce zinc sulfate and hydrogen gas.
(ii) Describe how hydrogen gas is collected during the experiment.
Ans: Hydrogen gas is collected by the downward displacement of water method. A test tube filled with water is inverted over the zinc granules in the beaker containing dilute sulfuric acid. As the reaction proceeds, hydrogen gas displaces the water in the test tube, and it collects in the test tube.
(iii) What happens when a burning splint is brought near the mouth of the test tube containing collected hydrogen gas?
Ans: When a burning splint is brought near the mouth of the test tube containing collected hydrogen gas, you will hear a pop sound. This pop sound indicates the presence of hydrogen gas, which is highly flammable and combustible.
(iv) Explain the chemical equation for the reaction between zinc and dilute sulfuric acid.
Ans: The chemical equation for the reaction is:
Zinc (Zn) + Dilute Sulfuric Acid (H2SO4) → Zinc Sulfate (ZnSO4) + Hydrogen Gas (H2)
(v) Why is it important to perform this experiment in a well-ventilated area or under a fume hood?
Ans: It is important to perform this experiment in a well-ventilated area or under a fume hood because the evolution of hydrogen gas can displace the air, leading to the accumulation of hydrogen, which is flammable. Adequate ventilation helps to disperse any potentially dangerous gas buildup and ensures safety in the laboratory.
Q2: Answer the following questions based on the diagram given below:
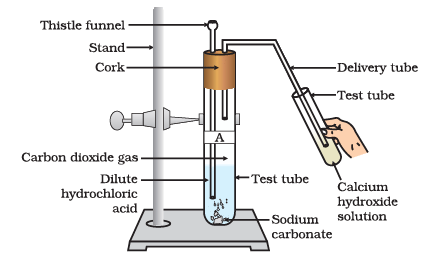
(i) What happens when carbon dioxide gas is passed through calcium hydroxide solution?
Ans: When carbon dioxide gas is passed through calcium hydroxide solution, it forms a white precipitate of calcium carbonate, which makes the solution turn milky.
(ii) What is the chemical equation for the reaction between carbon dioxide and calcium hydroxide solution?
Ans: The chemical equation for this reaction is:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
(iii) What is the common name for calcium carbonate?
Ans: Calcium carbonate is commonly known as limestone, chalk, or marble, depending on its form.
(iv) Why does the milky appearance of calcium hydroxide solution disappear when excess carbon dioxide is passed through it?
Ans: The milky appearance disappears when excess carbon dioxide is passed because calcium carbonate formed initially is slightly soluble in water. It dissolves in the excess carbon dioxide to form calcium bicarbonate, which is soluble.
(v) What type of reaction is represented by the reaction between calcium hydroxide solution and carbon dioxide?
Ans: The reaction between calcium hydroxide solution and carbon dioxide is a neutralization reaction, where a base (calcium hydroxide) reacts with an acid (carbon dioxide) to form a salt (calcium carbonate) and water.
Q3: Answer the following questions based on the diagram given below:
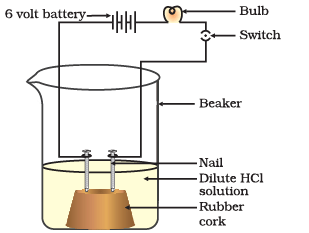 (i) What is the purpose of the experiment shown in the diagram?
(i) What is the purpose of the experiment shown in the diagram?
Ans:The purpose of this experiment is to demonstrate that an acid solution in water can conduct electricity.
(ii) In the experiment, what is represented by the two beakers filled with solutions?
Ans: The two beakers represent containers filled with different solutions. One contains an acid solution in water, and the other contains distilled water.
(iii) Explain why the bulb in the circuit glows when the wires are connected to the beaker containing the acid solution.
Ans:The bulb in the circuit glows when connected to the beaker with the acid solution because the acid solution allows the flow of electric current. This indicates that acids are conductors of electricity.
(iv) What happens when the wires are connected to the beaker containing only distilled water?
Ans:When the wires are connected to the beaker containing only distilled water, the bulb in the circuit does not glow. This shows that distilled water does not conduct electricity.
(v) How can you relate this experiment to real-life situations where understanding the conductivity of liquids is important?
Ans: This experiment helps us understand the conductivity of liquids, which is crucial in various real-life situations such as testing the purity of water, understanding the behavior of different substances in electrical circuits, and in the functioning of batteries and electrochemical cells. It has practical applications in science and industry.
Q4: Answer the following questions based on the diagram given below:
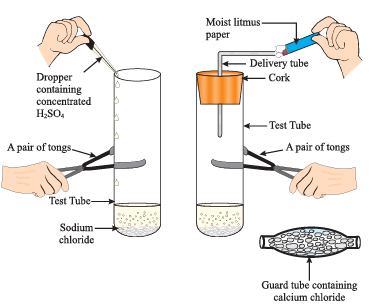
(i) What chemical reaction is being demonstrated in this experiment?
Ans: The experiment shows the reaction between concentrated sulfuric acid (H₂SO₄) and sodium chloride (NaCl), producing hydrogen chloride (HCl) gas.
(ii) What is the role of the moist litmus paper in the experiment?
Ans: The moist litmus paper is used to detect the presence of hydrogen chloride gas. When the gas comes in contact with the moist blue litmus paper, it turns red, indicating the acidic nature of the gas.
(iii) Why is a guard tube containing calcium chloride used in the experiment?
Ans: The guard tube containing calcium chloride is used to absorb any moisture present in the gas, ensuring that dry hydrogen chloride gas is tested.
(iv) What happens to the sodium chloride in the test tube when concentrated sulfuric acid is added?
Ans: When concentrated sulfuric acid is added to sodium chloride, a chemical reaction occurs, producing hydrogen chloride gas (HCl) and sodium bisulfate (NaHSO₄) as the products.
(v) Why is a pair of tongs used to handle the test tube in this experiment?
Ans: A pair of tongs is used to handle the test tube for safety, as the reaction can produce heat, and concentrated sulfuric acid is highly corrosive, so direct contact with the hands should be avoided.
Q5: Answer the following questions based on the diagram given below:
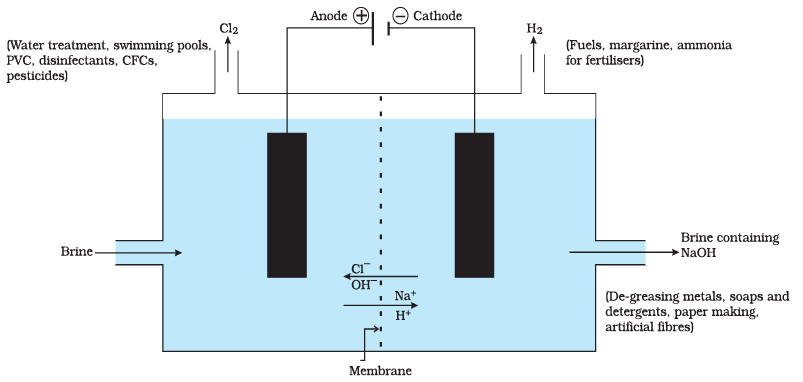
(i) What are the main products of the chlor-alkali process?
Ans: The main products of the chlor-alkali process are chlorine gas (Cl2), sodium hydroxide (NaOH), and hydrogen gas (H2).
(ii) How is chlorine gas produced in the chlor-alkali process?
Ans: Chlorine gas is produced at the anode during the electrolysis of brine (sodium chloride solution) in the chlor-alkali process.
(iii) What is the chemical equation for the production of sodium hydroxide in the chlor-alkali process?
Ans: The chemical equation for the production of sodium hydroxide is:
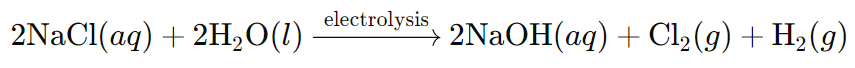
(iv) Why is the chlor-alkali process important in industry?
Ans: The chlor-alkali process is important in industry because it produces key chemicals like chlorine, sodium hydroxide, and hydrogen, which are used in various industrial processes, including water purification, manufacturing of soaps and detergents, and the production of plastics.
(v) What is the environmental impact of the chlor-alkali process, and how can it be mitigated?
Ans: The chlor-alkali process can generate chlorine gas, which is harmful to the environment. To mitigate its impact, modern industrial processes often use membrane cell technology that reduces the release of chlorine gas into the atmosphere, making the process more environmentally friendly.
Q6: Answer the following questions based on the diagram given below:
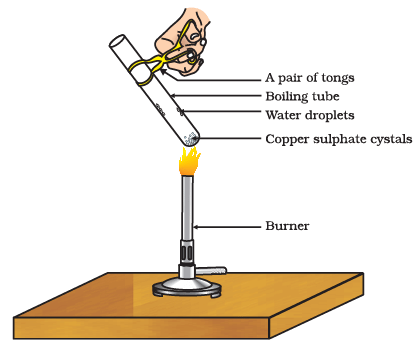
(i) What is water of crystallization?
Ans: Water of crystallization is a fixed number of water molecules that are chemically bound to the ions or molecules in a crystalline structure of a salt.
(ii) How can you visually tell if copper sulfate crystals contain water of crystallization?
Ans: You can tell if copper sulfate crystals contain water of crystallization by their blue color. Dry crystals are blue, indicating the presence of water molecules within the crystal lattice.
(iii) What happens to copper sulfate crystals when they are heated?
Ans: When copper sulfate crystals are heated, they lose their water of crystallization, and the salt turns from blue to white.
(iv) What is the chemical formula for hydrated copper sulfate?
Ans: The chemical formula for hydrated copper sulfate is CuSO4·5H2O, which means there are five water molecules for every formula unit of copper sulfate.
(v) Can you name another salt that has water of crystallization, and what is its chemical formula?
Ans: Yes, another salt with water of crystallization is gypsum. Its chemical formula is CaSO4·2H2O, which means it contains two water molecules for every formula unit of gypsum.
|
80 videos|569 docs|80 tests
|
FAQs on Diagram Based Questions: Acids, Bases and Salts - Science Class 10
| 1. What are some common examples of acids, bases, and salts? |  |
| 2. How do acids and bases differ in terms of their chemical properties? |  |
| 3. What is the pH scale and how does it relate to acids and bases? |  |
| 4. How are acids, bases, and salts used in everyday life? |  |
| 5. What are some common indicators used to test for acids and bases? |  |

















