What Happens to Nitrogen in Soils? - 2 | Agriculture Optional for UPSC PDF Download
Removal of Nitrogen from Soil
There are four primary mechanisms through which nitrogen is depleted from soils:
- Plant Uptake: This term refers to the absorption of nitrogen by plant roots. Different crops, such as cotton, corn, tomatoes, and turf grasses, require varying amounts of nitrogen, typically ranging from 60 to 300 pounds per acre, to achieve robust growth, profitable yields, or the desired aesthetic appearance. The specific nitrogen needs for a particular crop depend on its potential for production and are significantly influenced by climatic conditions.
- Commercial Nitrogen Fertilizer: Since most soils have a low availability of plant-accessible nitrogen, commercial nitrogen fertilizers are commonly used to meet nitrogen requirements. When the nitrogen requirement exceeds 150 pounds per acre, it is usually divided into two or more applications. It's important to note that only the portion of plant nitrogen within the harvested crop is removed from the field. The rest of the plant nitrogen is returned to the soil as plant residue, re-entering the nitrogen cycle as organic nitrogen, as depicted in Figure 1.
- Gaseous Loss of Nitrogen: Nitrogen loss through gaseous processes can occur via denitrification or ammonia volatilization. Denitrification involves the conversion of nitrate nitrogen (NO3-N) into gaseous nitrogen oxide (N2O) or elemental nitrogen (N2). This process is carried out by anaerobic bacteria, which do not require free oxygen, and it is often observed in wet or waterlogged soils.
- Runoff and Erosion: Nitrogen loss can also happen through the runoff of water and soil erosion.
- Leaching: Leaching is the process through which nitrogen is carried down through the soil by water, potentially reaching groundwater.
 Because denitrification is an anaerobic process, gaseous losses from typical, oxygen-rich (aerobic) soils are typically minimal. However, when soils remain consistently waterlogged or saturated for extended periods, a significant portion of the nitrate can be lost through this process.
Because denitrification is an anaerobic process, gaseous losses from typical, oxygen-rich (aerobic) soils are typically minimal. However, when soils remain consistently waterlogged or saturated for extended periods, a significant portion of the nitrate can be lost through this process.
Ammonia gas can be released from nitrogen compounds, such as urea, at the soil surface. Urea is found in animal manure and is also available for purchase in its pure form as a fertilizer with the composition 45-0-0.
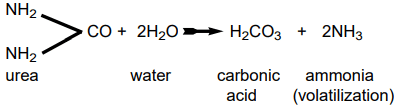
Various fertilizer compounds containing ammonium, such as ammonium sulfate (21-0-0), and, to a lesser extent, ammonium nitrate (33-0-0) and ammonium phosphate, have been observed to release free ammonia in the presence of calcium carbonate. This situation is particularly relevant in soils with a high pH level (pH>7.3).
Losses through runoff and erosion may encompass nitrate (NO3-), ammonium (NH4+), and organic nitrogen. The negatively charged NO3- ion remains in the soil water and is not bound by soil particles. If water carrying dissolved NO3- or NH4+ runs off the surface, these ions move along with it. However, when nitrogen fertilizers are applied to dry soils and then subjected to rain or irrigation, the initial water dissolves the fertilizer and carries it into the soil. In general, rainfall does not typically result in surface losses of fertilizer nitrogen, unless intense rainfall occurs shortly after application.
Ammonium held by clay particles can be transported into surface water supplies through soil erosion. Notably, soil erosion moves more nitrogen than rainfall does in terms of moving dissolved nitrogen compounds. When eroded soils are deposited into rivers and lakes, microbial processes gradually transform nitrogen compounds into soluble forms.
Leaching losses involve the downward movement of water through soil beyond the root zone. This primarily occurs with nitrate (NO3-) in regions characterized by high rainfall, excessive irrigation, and soils with a coarse texture (sandy soils). Leaching losses reduce the available nitrogen for crops and have the potential to contaminate shallow water wells and aquifers. Properly timing the application of nitrogen and adjusting the rates according to soil conditions and crop needs can help minimize leaching losses. Numerous research studies indicate that, due to plant uptake, minimal leaching of nitrate nitrogen (NO3--N) occurs in soils where crops are actively growing. While leaching is a more significant concern in sandy soils, which are prevalent in East Texas where grass is the primary crop, nitrogen fertilization is generally expected to result in minimal leaching losses statewide.
While limited issues have been identified in studies related to nitrate (NO3-) movement, inappropriate applications of both commercial and organic nitrogen fertilizers can lead to NO3- runoff into surface waters and leaching into groundwater.
Preventing Nitrogen Loss
The most effective approach to mitigate nitrogen losses from agricultural lands is by implementing sound soil and water management practices. The initial step in minimizing potential nitrogen losses involves soil testing. A correctly collected soil sample can provide an estimate of nitrate-nitrogen (NO3--N) content in the soil, serving as a reference for determining the appropriate amount of nitrogen fertilizer to apply for the specific crop being cultivated.
To prevent nitrogen from entering streams and lakes, the most effective strategies include proper fertilization practices and the control of surface runoff and erosion. When dealing with coarse-textured soils and regions with high levels of rainfall, leaching losses can be minimized by dividing the nitrogen requirement into multiple applications.
Implementing these practices ensures that nitrogen is used efficiently, reducing the risk of environmental contamination and promoting sustainable agriculture.
|
52 videos|224 docs
|
















