UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > F block: Magnetic, Spectral Properties & Separation Technologies
F block: Magnetic, Spectral Properties & Separation Technologies | Chemistry Optional Notes for UPSC PDF Download
Magnetic Properties
- The paramagnetic property of an ion or an atom is due to the presence of unpaired electrons in it. The magnetic properties of Lanthanides follow the above principle but there is a difference from ‘d’ block elements. The magnetic moments in 'd' block elemets are due to spin and orbital effects, of which the orbital effects, are usually quenched by the ligand field. This does not happen in lanthanides as 'f' orbitals are too deep inside the atom for such quenching to occur.
- Since La3+ (4f0, 5d0, 6s0), Ce4+ (4f0, 5d0 6s0) and Lu3+ (4f145d06s0), Yb2+ (4f145d06s0) have no unpaired electrons, they are diamagnetic but all the other lanthanide are paramagnetic.
- As mentioned above, the paramagnetic moments originates from spin and orgbital moments of the unpaired electrons in the atom or an ion. There are three possible modes of coupling between these components e.g. spin–spin, orbital–orbital and spin–orbital. For lanthanides al the three types of coupling are important, since for most of Ln3+ ions the energy difference between the two successive J levels of a multiplet (The multiplet width is large compared to KT where K is the Boltzman's constant and T is the absolute temperature), there is a strong L–S coupling. In these ions, the unpaired electrons in (n – 2) f orbitals are quite deeply seated and hence are well shielded by 5p and 5s electrons from the effect of other atoms in their compounds (Crystal Field Effect). Thus the effective magnetic moments of Ln3+ ions are
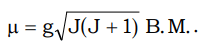
where J = Total angular momentum, which is obtained by L–S coupling or Russell – Saunder’s coupling.
g = Lande’s splitting factor or gyromagnetic ratio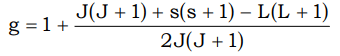
This is about 2.0023 - The spin orbit coupling constants are quite large, of the order of 1000 cm–1. This makes the seperation betwen the states with different J values much larger than KT (–200cm–1 at ordinary temp). This is the reflected in observed magnetic moments, which are in good agreement with those calculated from J values but differ widely from spin only values. J can have values as (L + S) (L + S – 1) ........................... (L – S) J = (L + S) when ‘f’ shell is more than half filled. J = (L – S) when ‘f’ shell is less than half filled.
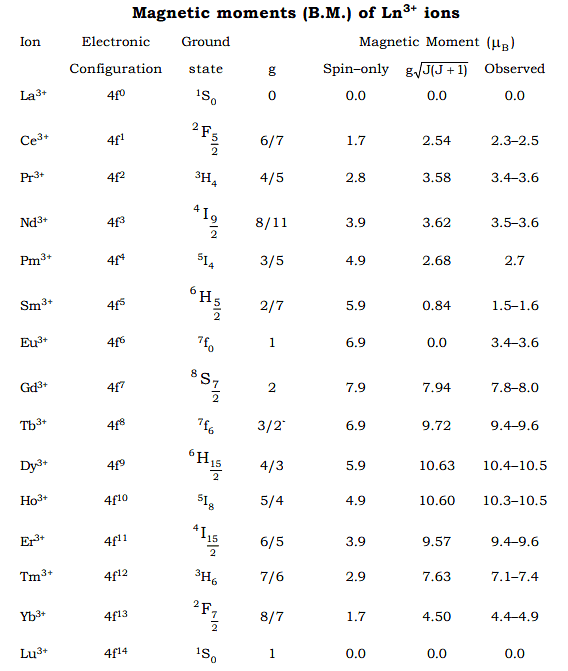
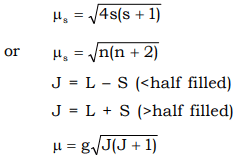
As it is clear from the above table, most of the lanthanide ions, there is good agreement between the experimental value and calculated value. For Sm3+ and Eu3+ the agreement is poor. As forJo → J1 transition in Sm3+ and Eu3+, hv = KT there is no simple expression for μ , the magnetic moment which gets contributions from some of the lower excited states of ions as well as from the ground state. Alternatively it can also be explained as below. - The seperation between the ground state and first excited state (ΔE) for these ions and the fvalues of spin–orbits coupling constant (λ) are
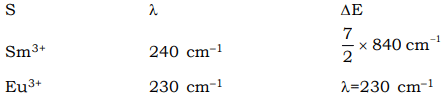 Comparison with the values of –200 cm–1 for KT at ordinary temperature leads us to conclude that the first two or three excited state of Sm3+ and Eu+3 will be populated. Mixing of these states with higher J values causes the observed magnetic moment to be higher.
Comparison with the values of –200 cm–1 for KT at ordinary temperature leads us to conclude that the first two or three excited state of Sm3+ and Eu+3 will be populated. Mixing of these states with higher J values causes the observed magnetic moment to be higher.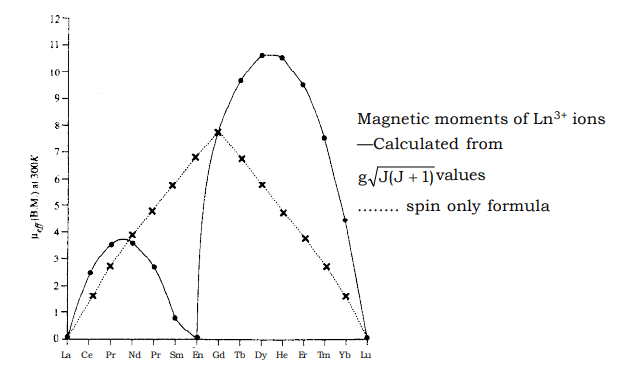 The two peaks arise from the fact that with orbitals less than half filled, the components tend to oppose each other while orbitals more than half fille, they reinforce each other (Hund’s third rule). At f7, there is no orbital angular momentum thus Gd3+ has a lower magnetic moment than Dy3+ and Ho3+ though it has maximum unpaired electrons. In the case of Eu3+, there are 6 unpaired electrons, but as the spin and orbital moments are equal and opposite, the net calculated moment is zero as shown in the graph.
The two peaks arise from the fact that with orbitals less than half filled, the components tend to oppose each other while orbitals more than half fille, they reinforce each other (Hund’s third rule). At f7, there is no orbital angular momentum thus Gd3+ has a lower magnetic moment than Dy3+ and Ho3+ though it has maximum unpaired electrons. In the case of Eu3+, there are 6 unpaired electrons, but as the spin and orbital moments are equal and opposite, the net calculated moment is zero as shown in the graph. - At low temperature this has been proved experimentally but at a higher temperature it shows paramagnetism. Same is the case in Sm3+ also. The magnetic behaviour of actinides are vey complicated. The values of magnetic moments found experimentally are usually lower than those calculated using Rusell – Saunder’s coupling sheme. This may be due to the inadequacy of Russell Saunder’s coupling scheme for 5f ions and also due to the quenching of orbital contribution by crystal field effects which involve 5f orbitals to a greater extent than the 4f orbitals involved in lanthanides.
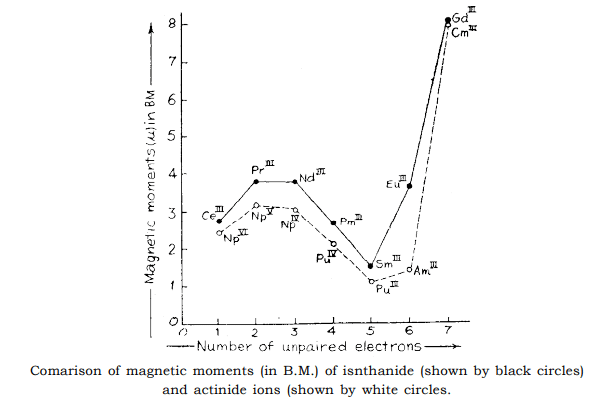
- The graph gives a comparison of magnetic moments in Bohr Magnetons (B.M.) of lanthanides and actinide ions. The moments of the lanthanide ions agree closely with theoritical predictions as shown earlier, but those of the transuranic ions are some what lower than expected. This is because the 5f electrons of transuranic elements ions are less effectively screened from the crystal field which quenches the orbital contribution than the 4 f orbital of lanthanide ions.
Question for F block: Magnetic, Spectral Properties & Separation Technologies
Try yourself:
Which type of coupling is important for most of the Ln3+ ions?View Solution
Spectral Properties
- The aqueous solutions of most lanthanide ions are coloured except when the ions have f0, f1, f7, f13 or f14 configurations.
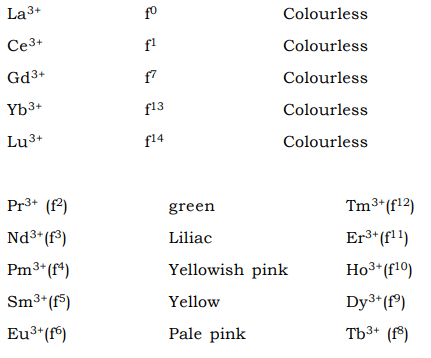
- From the above chart it is clear that ions with fn and f14 – n configurations have similar colours. The colours are produced by the absorption of electromagnetic radiation in the visible region corresponding to the transition of the ions from their ground states to excited states. For the lanthanide ions the excited states are from the same 4f or in other words the colours in Lanthanide ions are due to Lapore’s forbidden f–f transition like the d–d transition. However the relaxation available in d–d transitions owing to distortion from crystal fiel d effects is much less pronounced for the deep seated 4f orbitals.
- Hence the absorption bands of Ln3+ are very weak but sharp when compared to those of ‘d’ block elements. Many of the bands are line like and become even narrower as the temperature is lowered. These bands are independent of the nature of anions present. The narrowness of the bands indicate that electronic transitions do not excite much mol,ecular vibrations when it occurs, ‘f’ electrons interact weakly with the ligands. Thus, the excited electrons interacts only weakly with the environment, the non radioactive life time of the excited state is quite long and luminisense can be expected. Thus these are used as phosphors in T.V. screen.
- Though the basic principles underlying the spectral properties in ‘d’ block elements and ‘f’block elements are same, there are certain important differences.
(a) The ‘f’ orbitals lie deep inside and as mentioned earlier, are independent by the nature of ligands and thermal vibrations. Thus the bands from f–f transitions are sharp but those from d–d transitions are broad due to environmental effects.
(b) Spin–orbit coupling for the lanthanides are more important than crystal field effect.
(c) The number of theoritically possible vibrations are large in lanthanides, giving rise to number of sharp bands as shown below.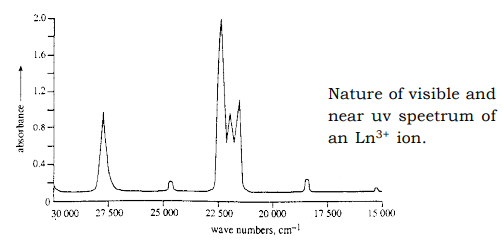 (d) Though the crystal field effects in lanthanides are small as compared to ‘d’ block elements, fine structures are observed as shown in the above graph. This is due to the effect of some coordinated ligands.
(d) Though the crystal field effects in lanthanides are small as compared to ‘d’ block elements, fine structures are observed as shown in the above graph. This is due to the effect of some coordinated ligands. - Laport’s permitted bands are due to the transition of 4f—5d. This has been observed in Ce3+, Tb3+, Sm2+, Eu3+ and Yb2+. These bands are stronger and broader as the transitions are influenced by the chemical environments.
- Charge Transfer Spectra is also observed in certain lanthanide ions. Lanthanides in higher oxidation state e.g Ce4+ show orange red colour due to L → M transition (LMCT) Compounds of Eu3+ with reducing anions are yellow due to electron transfer from metal to ligand (MLCT). Compounds of lanthanides have a large range of applications due to f–f electronic transitions. Europium oxide and Europium orthovanadate are used as phosphors in televisions and computer terminal displays. Neodynium (Nd3+), Samarium (Sm3+) and Holmium (Ho3+) are used in solid state lasers.
- The spectra of Actinides are similar to those of lanthanides but are more complicated because of the following reasons.
(a) Russell–Saunders coupling scheme is inefficient in heavier actinides.
(b) The 5‘f’ electrons have greater penetrating effect and come closer to the nucleus.
(c) The improper shielding of 5‘f’ electrons from the surrounding chemical environment leads to ligands effects.
Since 5‘f’, 6‘d’ and 7‘s’ electrons have comparable energies, the electronic configuration of the element in a given oxidation state in solution depends on the ligand. This property is known as ligand effect. This is not shown by lanthanides. - Actinides show Laporte’s forbidden 5f → 5f transition and Laporte’s allowed 5f → 6d transition resulting in absorption spectra besides charge transfer spectra. The f—f transitions in actinides are about 10 times more intense and the ligand field effect is about 2 to 5 times stronger than in other lanthanides. The elements with same number of ‘f’ electrons is 4f (lanthanides) and 5 ‘f’ (actinides) have comparable spectra, as shown below.
 Cations having 5f0, 5f7 and 5f14 are colourless as in lanthanides. The absorption spectra of actinides in aqueous solution and in crystals, are in visible, near UV and near 1R regions. Charge Transfer (CT) spectra occur more frequently in actinides and the bands are more intense because of lower energy involved in transitions. Morever the overlap of 5‘f’ orbitals with the ligand orbitals increases the intensity of absorption bands. In short, the absorption spectra of actinides can be divided into two groups.
Cations having 5f0, 5f7 and 5f14 are colourless as in lanthanides. The absorption spectra of actinides in aqueous solution and in crystals, are in visible, near UV and near 1R regions. Charge Transfer (CT) spectra occur more frequently in actinides and the bands are more intense because of lower energy involved in transitions. Morever the overlap of 5‘f’ orbitals with the ligand orbitals increases the intensity of absorption bands. In short, the absorption spectra of actinides can be divided into two groups.
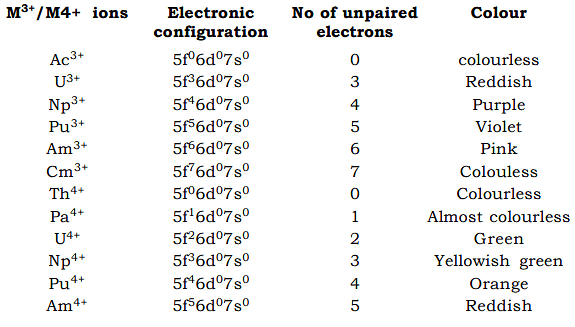
(a) Am3+ and heavier actinides which have spectra resembling those of lanthanides.
(b) Pu3+ and lighter actinides which resemble lanthanides spectra in some ways but have a tedendency of broadening of absorption peaks which is seen in transition metal spectra. - The greater exposure of 5‘f’ orbitals in the lighter actinide elements results in greater ligand–metal orbital interaction and some broadening results from the 5‘f’ orbitals behave more like transition metals and the spectra of heavier actinides resemble those of lanthanides.
Question for F block: Magnetic, Spectral Properties & Separation Technologies
Try yourself:
Which method is most commonly used for the separation of lanthanides based on the poor shielding of 4f electrons?View Solution
Separation Techniques in Lanthanides
 |
Download the notes
F block: Magnetic, Spectral Properties & Separation Technologies
|
Download as PDF |
Download as PDF
(a) Ion Exchange
- The most rapid and effective procedure for the seperation of lanthanides is Ion Exchange method. This method is based on the fact that because of poor sheilding of 4‘f’ electrons, the size of Ln3+ ions decrease from La3+ to Lu3+ (lanthanide contraction). Because of this, the binding of Ln3+ ions to a complexing agent gradually and regularly increases with increasing atomic number of lanthanides resulting in the increase of formation constant from La3+ to Lu3+. When the complexes thus formed is eluted with a elutant, the lanthanide ions will be eluted in a sequence from higher atomic number to lower atomic number (Lu3+ to La3+) as shown in the graph.
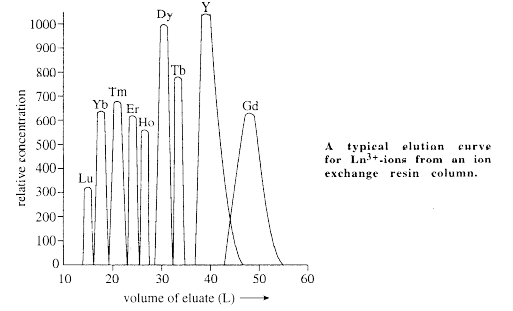
- There are a number of cation exchange resins like Domex–50, Sodium polystyrene sulphonate etc where Ln3+ ions are absorbed on the resin bed replacing the Hydrogen in –SO3H groups (resin–R) or Na+ in Sodium polystyrene sulphonate.
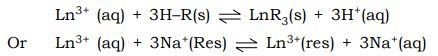
The experimental details with the later cation exchange resin is given below.
A solution of Ln3+ ions is introduced at the top of a cation exchange column in the sodium polystyrence sulphonate. The Ln3+ ions readily undergo ion exchange displacing Na+ ions forimg a band of lanthanide ions bound to the top of the cation exchange column. To move these ions down the column, a solution of anionic ligand e.g. citrate, tartarate, lactate, etc. is slowly passed through the column. These anionic chelating ligands form complexes with lanthanides.
As these complexes possess a lower positive charge than Ln3+ they are less tightly held by the resin than Ln3+ and are displaced from the ion exchange material into the surrounding solution.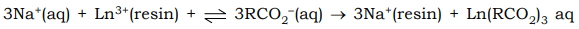
The Ln3+ cations with smallest radius are most strongly bound to the anionic ligand, so these ions have the greatest tendency to be eluted first. - The efficiency of the ion exchange method is enhanced by “displacement chromatography”. In this method the eluted solution is passed through a second column charged with Cu(II) or Zn(II) on the resin bed. The smaller Cu2+ ion on the resin bed is displaced by Ln3+ ion which is deposited in a compact bond. The Ln3+ ions are than eluted from the resin bond.
- From the solutions obtained from ion exhange columns, the lanthanides are precipitated as fluorides or hydroxides or oxalates. The hydraoxides and oxalates yield oxides on heating, which is converted to chloride by heating with NH4Cl or CCl4.
(b) Solvent Extraction Method
- This method is based on the differences in the solubility of lanthanides salts in water and an immiscible or partially miscible organic solvent. The organic solvents are called extracting solvents or extractants. This method can be used as tracer or in macrosales.
- In this method, the aqueous solution of lanthanide salts is passed through the organic solution which extracts the lanthanides from water. Tn–n–butyl phosphate (TBP) is the most commonly used solvent for extraction of lanthanides from dilute nitric acid solution. The solubility of Lu(III) elements increases slightly with increasing atomic number. The efficiency of the process may be increased by employing continuous counter current procedure which enables large number of partions suitable for large scale production.
- Bis 2 ethyl hexyl phosphinic acid (DEHPA) is now largely used as it has a higher seperation factor.
- The details of the solvent extraction technique is given below. When TBP is used as extracting solvent and aqueous solution of lanthanide salts are passed through it, the TBP form complexes with the Lu3+(aq) ions in presence of NO3 – ions.
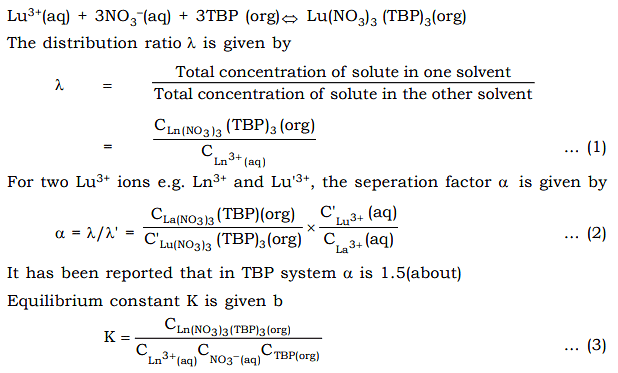
Combining (1) and (3)
95% pure lanthanides can be seperated by this method. However this method cannot compete with Ion Exchange method. This method is mainly used to seperate Ln3+ from Lu4+ ions,e.g. Ce4+ and Th4+.
The document F block: Magnetic, Spectral Properties & Separation Technologies | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on F block: Magnetic, Spectral Properties & Separation Technologies - Chemistry Optional Notes for UPSC
| 1. What are the magnetic properties of the lanthanides? |  |
| 2. How do the spectral properties of lanthanides contribute to their unique characteristics? |  |
Ans. The spectral properties of lanthanides refer to their ability to emit or absorb light at specific wavelengths. This property is utilized in various applications such as fluorescence imaging, laser technology, and colorimetry. The unique energy levels and transitions of lanthanide ions allow for specific emission or absorption of light, making them valuable in these fields.
| 3. What are the separation techniques commonly used for lanthanides? |  |
Ans. Lanthanides can be separated using various techniques such as solvent extraction, ion exchange chromatography, and precipitation. Solvent extraction involves the selective dissolution of lanthanides in a suitable organic solvent. Ion exchange chromatography utilizes a solid stationary phase to separate lanthanides based on their charge and size. Precipitation involves the formation of insoluble compounds to separate lanthanides from a solution.
| 4. How do the magnetic properties of lanthanides affect their separation using magnetic techniques? |  |
Ans. The magnetic properties of lanthanides can be utilized in magnetic separation techniques. Lanthanides with high magnetic moments can be separated using magnetic fields. By applying a magnetic field, the lanthanides can be attracted or repelled, allowing for their separation from a mixture. This technique is particularly useful when dealing with paramagnetic lanthanides.
| 5. How can the spectral properties of lanthanides be utilized in practical applications? |  |
Ans. The spectral properties of lanthanides have various practical applications. For example, lanthanide ions are used as dopants in phosphors, which emit specific colors when excited by an energy source. This is utilized in the production of fluorescent lamps, cathode ray tubes, and television screens. Lanthanides are also used in the development of lasers, where their specific emission wavelengths are important for precise and efficient laser operation.
Related Searches





















