IUPAC Nomenclature of Coordination Complexes | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Introduction |

|
| Rule 1: Anionic Ligands |

|
| Rule 2: Neutral Ligands |

|
| Rule 3: Ligand Multiplicity |

|
| Rule 4: The Metals |

|
| Writing Formulas of Coordination Complexes |

|
Introduction
According to the Lewis base theory, ligands are Lewis bases since they can donate electrons to the central metal atom. The metals, in turn, are Lewis acids since they accept electrons. Coordination complexes consist of a ligand and a metal center cation. The overall charge can be positive, negative, or neutral. Coordination compounds are complex or contain complex ions, for example:
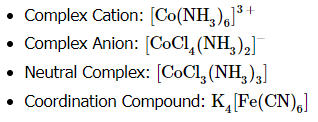
A ligand can be an anion or a neutral molecule that donates an electron pair to the complex (NH3, H2O, Cl-). The number of ligands that attach to a metal depends on whether the ligand is monodentate or polydentate. To begin naming coordination complexes, here are some things to keep in mind.
- Ligands are named first in alphabetical order.
- The name of the metal comes next.
- The oxidation state of the metal follows, noted by a Roman numeral in parentheses (II, IV).
Rule 1: Anionic Ligands
Ligands that act as anions which end in "-ide" are replaced with an ending "-o" (e.g., Chloride → Chloro). Anions ending with "-ite" and "-ate" are replaced with endings "-ito" and "-ato" respectively (e.g., Nitrite → Nitrito, Nitrate → Nitrato).
Table 1: Anionic Monodentate Ligands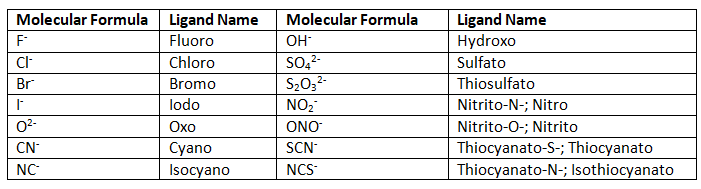
Rule 2: Neutral Ligands
Most neutral molecules that are ligands carry their normal name. The few exceptions are the first four on the chart: ammine, aqua, carbonyl, and nitrosyl.
Table 2: Select Neutral Monodentate Ligands. Note: Ammine is spelled with two m's when referring to a ligand. Amines are a class of organic nitrogen-containing compounds.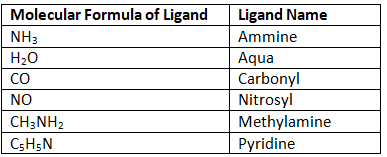 Polydentate ligands follow the same rules for anions and neutral molecules.
Polydentate ligands follow the same rules for anions and neutral molecules.
Table 3: Select Polydentate ligands
Rule 3: Ligand Multiplicity
The number of ligands present in the complex is indicated with the prefixes di, tri, etc. The exceptions are polydentates that have a prefix already in their name (en and EDTA4- are the most common). When indicating how many of these are present in a coordination complex, put the ligand's name in parentheses and use bis (for two ligands), tris (for three ligands), and tetrakis (for four ligands).
Table 4: Prefixes for indicating number of ligands in a complex.
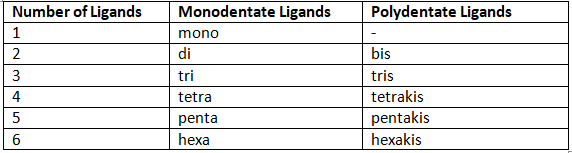
Prefixes always go before the ligand name; they are not taken into account when putting ligands in alphabetical order. Note that "mono" often is not used. For example, [FeCl(CO)2(NH3)3]2+ would be called triamminedicarbonylchloroiron(III) ion. Remember that ligands are always named first, before the metal is.
Solved Examples
Example 1: What is the name of this complex ion: [CrCl2(H2O)4]+?
Ans: Let's start by identifying the ligands. The ligands here are Cl and H2O. Therefore, we will use the monodentate ligand names of "chloro" and "aqua". Alphabetically, aqua comes before chloro, so this will be their order in the complex's name. There are 4 aqua's and 2 chloro's, so we will add the number prefixes before the names. Since both are monodentate ligands, we will say "tetra[aqua]di[chloro]".
Now that the ligands are named, we will name the metal itself. The metal is Cr, which is chromium. Therefore, this coordination complex is called tetraaquadichlorochromium(III) ion. See the next section for an explanation of the (III).
Example 2: What is the name of this complex ion: [CoCl2(en)2]+?
Ans: We take the same approach. There are two chloro and ethylenediamine ligands. The metal is Co, cobalt. We follow the same steps, except that en is a polydentate ligand with a prefix in its name (ethylenediamine), so "bis" is used instead of "di", and parentheses are added. Therefore, this coordination complex is called dichlorobis(ethylenediamine)cobalt(III) ion.
Rule 4: The Metals
When naming the metal center, you must know the formal metal name and the oxidation state. To show the oxidation state, we use Roman numerals inside parenthesis. For example, in the problems above, chromium and cobalt have the oxidation state of +3, so that is why they have (III) after them. Copper, with an oxidation state of +2, is denoted as copper(II). If the overall coordination complex is an anion, the ending "-ate" is attached to the metal center. Some metals also change to their Latin names in this situation. Copper +2 will change into cuprate(II). The following change to their Latin names when part of an anion complex:
Table 5: Latin terms for Select Metal Ion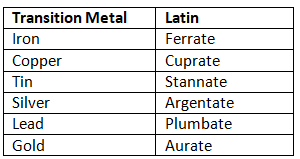
The rest of the metals simply have -ate added to the end (cobaltate, nickelate, zincate, osmate, cadmate, platinate, mercurate, etc. Note that the -ate tends to replace -um or -ium, if present).
Finally, when a complex has an overall charge, "ion" is written after it. This is not necessary if it is neutral or part of a coordination compound (Example 3). Here are some examples with determining oxidation states, naming a metal in an anion complex, and naming coordination compounds.
Solved Examples
Example 1: What is the name of [Cr(OH)4]-?
Ans: Immediately we know that this complex is an anion. There is only one monodentate ligand, hydroxide. There are four of them, so we will use the name "tetrahydroxo". The metal is chromium, but since the complex is an anion, we will have to use the "-ate" ending, yielding "chromate". The oxidation state of the metal is 3 (x+(-1)4=-1). Write this with Roman numerals and parentheses (III) and place it after the metal to get tetrahydroxochromate(III) ion.
Example 2: What is the name of [CuCl4]2−?
Ans: tetrachlorocuprate(II) ion
Note: When naming a coordination compound, it is important that you name the cation first, then the anion. You base this on the charge of the ligand. Think of NaCl. Na, the positive cation, comes first and Cl, the negative anion, follows.
Example 3: What is the name of [Pt(NH3)4][Pt(Cl)4]?
Ans: NH3 is neutral, making the first complex positively charged overall. Cl has a -1 charge, making the second complex the anion. Therefore, you will write the complex with NH3 first, followed by the one with Cl (the same order as the formula). This coordination compound is called tetraammineplatinum(II) tetrachloroplatinate(II).
Example 4: What is the name of [CoCl(NO2)(NH3)4]+?
Ans: This coordination complex is called tetraamminechloronitrito-N-cobalt(III). N comes before the O in the symbol for the nitrite ligand, so it is called nitrito-N. If an O came first, as in [CoCl(ONO)(NH3)4]+, the ligand would be called nitrito-O, yielding the name tetraamminechloronitrito-O-cobalt(III).
Nitro (for NO2) and nitrito (for ONO) can also be used to describe the nitrite ligand, yielding the names tetraamminechloronitrocobalt(III) and tetraamminechloronitritocobalt(III).
Writing Formulas of Coordination Complexes
While chemistry typically follow the nomenclature rules for naming complexes and compounds, there is disagreement with the rules for constructing formulas of inorganic complex. The order of ligand names in their formula has been ambiguous with different conventions being used (charged vs neutral, number of each ligand, etc.). In 2005, IUPAC adopted the recommendation that all ligand names in formulas be listed alphabetically (in the same way as in the naming convention) irrespective of the charge or number of each ligand type.
However, this rule is not adhered to in many chemistry laboratories. For practice, the order of the ligands in chemical formulas does not matter as long as you write the transition metal first, which is the stance taken here.
Solved Examples
Example 1: Write the chemical formulas for:
- Amminetetraaquachromium(II) ion
- Amminesulfatochromium(II)
Ans:
- Amminetetraaquachromium(II) ion could be written as
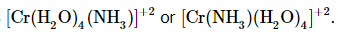
- Amminesulfatochromium (II) could be written as
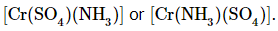
Example 2: Write the chemical formulas for
- Amminetetraaquachromium (II) sulfate
- Potassium hexacyanoferrate (III)
Ans:
- Amminetetraaquachromium (II) sulfate can be written as
 is also acceptable.
is also acceptable. - Potassium hexacyanoferrate (III) is be written as K3[Fe(CN)6]
FAQs on IUPAC Nomenclature of Coordination Complexes - Chemistry Optional Notes for UPSC
| 1. What are anionic ligands? |  |
| 2. How are neutral ligands different from anionic ligands? |  |
| 3. What is ligand multiplicity? |  |
| 4. How do you write formulas of coordination complexes? |  |
| 5. What is the IUPAC nomenclature of coordination complexes? |  |














